Search
-
Department Spotlight Clinical Research
May 20 is National Clinical Trials Day. Celebrate with us by recognizing the Clinical Research team at Renown Health! The root of every medication, treatment and procedure in healthcare can be traced back to research. From the beginning of the history of medicine, research has always played a crucial role in improving the lives of patients around the world, leaving a permanent mark on how we expand our medical capabilities to this day. Renown Health’s Clinical Research team, in partnership with the University of Nevada, Reno School of Medicine (UNR Med), are leading that effort in our very own community. As our in-house leaders of clinical trials, this team is dedicated to advancing the science of medicine to help further our bottom line of making a genuine difference in the health and well-being of the patients they serve. Trial by (Medical) Jury Every day looks different for the Clinical Research team, especially when it comes to interacting with patients, providers and “sponsors,” which are the organizations providing the treatment for the study. One fact always remains true: communication and collaboration are key, especially among the team who act as the face of this process. Meet Lisa English (pictured above on the far right in a blue shirt), a Lead Clinical Research Coordinator at Renown who serves as the study "project manager." One aspect of Lisa’s day-to-day is seeing patients through their clinical trials from start to finish. It all begins with the setup. “Before we can launch a study, there is a lot of back-and-forth dialogue between everyone involved to ensure the best fit,” said Lisa. “Sponsors will come to us with novel treatments, such as medications or devices, and the inclusion criteria that patients need to meet in order to qualify for the study. We then immediately jump into working with the providers, looking closely at the science and comparing the treatments to what is on the market already.” From there, Lisa coordinates conversations between the providers, sponsors and study teams to gauge everyone’s capacity based on the length of the study, ensuring everyone involved has the time to dedicate to the process. Next, the providers identify patients that meet the criteria for the study, and together, the team decides where the patient visits will happen and discusses any potential barriers that may affect patient retention. The budget is clearly defined at this stage, set up to make sure no patient is ever billed for medical costs incurred as a result of the study. Often, research participants are seen within the specialty clinics throughout the health system, while other times the Clinical Research Coordinators see patients at the recently centralized Clinical Research Office at Renown Regional Medical Center. This location provides an essential public-facing space for the community to learn more about clinical trials and demonstrates the breadth of resources available at Renown to sponsors to strengthen external partnerships and research funding opportunities. Once the study officially begins, team members like Lisa will set patients up for a “screening/qualifying visit.” During this appointment, she makes sure patients get scheduled for their lab work, imaging scans and anything else the provider may need to make an executive decision on whether or not the patient is a good candidate for the study. “I build it all in Epic,” said Lisa. “The study information, directions, requirements and next steps are all loaded in Epic for easy tracking. We are also required to input any notes in the sponsor’s electronic data capture website. All the information I track is inputted without protected health information (PHI), so every patient is completely anonymous.” After the patient officially qualifies, the study goes full steam ahead. Team members like Lisa and the providers receive continual updates from sponsors on the status of the study. “Throughout the entire process, I make sure patients get scheduled for everything that meets the requirements for the study,” said Lisa. “I meet with patients one-on-one to discuss their needs and concerns and ask questions about the study, organize their appointments and charts and deal with any issues or pivots that may arise. It’s very important that every patient fully understands what is going to happen with their care.” The Clinical Research department strives to serve as a care partner to patients, providers and clinics they work with. The majority of our Clinical Research Coordinators are trained phlebotomists and medical assistants, performing their own assessments such as lab draws and electrocardiograms (ECGs) to streamline the research visit process and reduce resource constraints on the clinics and health system. Lisa typically sees a couple of patients per week, depending on the study and where patients are in the cycle. Depending on the complexity of the trial, patients may see the research team only one time or several times over many years. Typically, clinical trial patients are seen in clinic every 2-4 weeks. There are many tasks required before, during and after a research visit to ensure everything runs smoothly, so Clinical Research Coordinators dedicate an average of 5-11 hours of work per patient, per visit. Regardless of patient load, each employee in the Clinical Research department – as well as participating teams across Renown and UNR Med – always step in to help each other out. According to Lisa, the environment is immensely supportive. “We have a program here at Renown to train employees who have never done clinical trials,” said Lisa. “We love seeing people get more engaged with the important work we do, and every department has been great at collaborating with us. Everyone brings a different perspective.” At the end of the study, Lisa gathers all the information and collects notes into a zip drive or paper binder for archiving. The sponsor lets the Clinical Research team, providers and patients know whether they are on the trial drug or on the placebo. The teams use the data gathered during the study to publish a report or present at conferences, promoting the critical research done to better the lives of patients in our community, and potentially, the world. “I appreciate the time everyone gives us to make sure our research is successful,” said Lisa. “It feels great to work together to make a difference, improve healthcare quality and save lives.” Behind-the-Scenes, Yet on the Frontlines The impact of research studies transcends hospital walls, and this can all be attributed to the dedication of our Clinical Research department. The constant collaboration between this team, lab science, medical assistants and providers, cardiology technologists, sonographers, finance teams and our partners at UNR Med is crucial to safeguarding the success of the studies. Devoted to keeping research close to home, Renown and UNR Med teamed up to form the Clinical Research Office (CRO) in 2021. With the strength of northern Nevada's largest not-for-profit health system and Nevada’s first medical school, this team is dedicated to giving our community access to the latest care innovations. “At UNR Med, we are working with students, residents and academic faculty; on the Renown side, we are working with clinicians and community participants,” said Amber Emerson, Manager for Community Outreach and Research Engagement for UNR Med. “Everything we do is data-driven,” added Kristen Gurnea, Manager of Clinical Research for Renown. “Our main goal is to optimize our impact and provide a community benefit for our patients. The scope of our roles in the Clinical Research office is very diverse.” To help meet the growing need locally for healthcare and cutting-edge treatment solutions, the CRO has continued to grow, expanding its research capabilities and helping bring new medications, medical devices and more to patients across northern Nevada and northeastern California. “Once upon a time, our team had only six members; today we have grown to a team of 25,” added Diana Torres, Research Resource Analyst for Renown. “We used to be considered one department, including Medical Education, and we have since branched off into our own cost center. We branched off even further and created a separate Genetics department that runs the Healthy Nevada Project. Throughout this process, the Clinical Research department was always the main point of the umbrella.” “We participate in hospital-wide outreach and marketing, and we feel this has really helped us get the word out about our department,” added Raul Arellano, Research Resource Analyst for Renown. “In fact, we doubled our clinical trial portfolio from last year.” The CRO currently operates over 100 clinical trials locally in cardiology, endocrinology, infectious disease, neurology, pediatric and adult oncology, pediatric sub-specialties and pulmonology. Behind the curtains of in-person research, the CRO is home to several experts who help turn our research studies into a reality, from budgeting and billing to barrier-breaking and building relationships. “I help with barriers patients and Clinical Research Coordinators are facing, building connections and relationships inside and outside of our health system,” said Kristen Gurnea. “I enjoy handling all the supporting pieces that are required for studies to happen.” “My role changes every day,” added Jenna Berger, Administrative Assistant for the CRO at Renown. “Some days, I’ll be helping coordinate patient stipends and going through document management to ensure we have all necessary signatures. Other days, I will be planning events – like Clinical Trials Week – for our department and creating marketing materials and fliers.” “Our day-to-day involves going over anything related to research financials,” added Diana Torres. “We handle sponsor billings, process efficiency and collecting revenue for research contracts, and we collaborate closely with our Finance department and Revenue Integrity in order to accomplish this. It’s important for us to make sure all billing on both the sponsor and patient side is taken care of, especially because patients should never receive a bill for medical services they receive for the trial. A year and a half ago, we started doing budget negotiations for research contracts,” said Diana Torres. “We are proud to help clinical teams with any training they may need on these negotiations as well as billing reviews and allocations.” Seeing patients progress during a study and transform before their eyes inspires the CRO team to continue doing what they do every day. “I’ve been here for many years, first working on the floor as an oncology nurse and transitioning to oncology research in 2005,” said Anna Winchell, Cancer Protocol Nurse for Renown. “I love getting to know the patients and seeing them progress into a healthy lifestyle.” Medical students and residents at UNR Med also play a significant role in the research process, advancing medicine by exploring causes and novel treatments for a wide range of conditions, including HIV, muscular dystrophy, gastrointestinal disorders, infectious diseases and more. Medical research at UNR Med is headed by committed research coordinators, community outreach managers, grants managers, pharmacists and physicians. “I oversee scientific review and help the physicians that come to us for those resources,” said Amil Trujillo-King, Medical Research Coordinator at UNR Med. “I guide medical students in their research protocols and help with different projects to improve research activities for both students and medical residents.” It takes a village to make clinical research happen. Because of that, the ACRO cannot thank the following teams enough for moving mountains for the future of medicine: Renown Health and UNR Med leadership for demonstrating the integrated health system’s commitment to expanding access to clinical research in our community within both the Renown / UNR Med affiliation and Renown active strategic plans. Renown Pharmacy especially Research Clinical Pharmacist Tim Morton, who supports all clinical trial medication dispensing and patient education across all clinical trials at Renown. Accounts Payable for having a huge impact on patient and employee reimbursement. Renown Medical Group for their participating providers, especially in oncology, cardiology, pulmonology, pediatrics, endocrinology and neurology, who are involved in research year after year. Marketing and Communications for helping with printed materials and raising awareness for clinical research at Renown and UNR Med. An Affiliation to Last Through the Ages A collective, shared vision of exploring community health – that is the impetus behind the affiliation between Renown and UNR Med. By leveraging resources across both institutions, the CRO has maximized their impact, giving the people of northern Nevada greater access to new interventions and treatments and promoting an impassioned culture with patients, providers, residents and medical students. “Community-based research always sat well with me,” said Amber Emerson. “As Renown and UNR Med, we have this unique opportunity to shape clinical research here in northern Nevada. We always make sure we present research in a meaningful way that speaks to the work we produce and demonstrates the opportunities we offer. After all, participating in clinical research doesn’t mean our patients are ‘guinea pigs’ – quite the opposite! They are partners in their health care, and we support them through providing access to novel treatments.” “Research is my passion, and my career has spanned broadly from grants administration to study coordination,” added Valerie Smith, Clinical Research Center Administrative Manager at UNR Med. “I am excited to be at the forefront of research frontiers in northern Nevada.” Through robust engagement and collaboration with healthcare providers, department administrators, internal research team members and leadership, the strength of this affiliation is unmeasurable. The CRO’s ultimate goal is to have clinical trials be the standard of care for every condition that Renown and UNR Med treats. Clinical research participation is all about patient autonomy, shared decision-making between patients and their providers and advancing medicine to save lives. From their beginnings as a small group of passionate researchers to their present reality as a leader in the research space in northern Nevada, their efforts do not go unnoticed. “The success of our department is inspiring,” said Amil Trujillo-King. “Renown and UNR Med supports the wellbeing of all employees and contributes directly to the growth of the department.” “When I first joined Renown in Patient Access, I didn’t realize that we had a research department; with a strong healthcare background in my family, I knew I wanted to grow in my career, and our expanding Clinical Research office was that next step,” said Raul Arellano. “With our affiliation with UNR Med, it’s especially inspiring to be able to apply what I learned as a Patient Access Representative to help further outcomes for our patients through managing our finances.” Through their unwavering commitment to research excellence and patient-centered care, the CRO will continue to pave the way for groundbreaking medical discoveries and improved outcomes for patients for years to come. “Fundamentally, we’re working to build a culture of research in our community because we believe it is the right thing to do. Our community deserves to have access to clinical trials and novel care close to home with a dedicated team to support them every step of the way,” closes Kristen Gurnea.
-
Monkeypox: A Renown Expert Weighs In
Renown Health is closely following the national outbreak of the monkeypox virus and urging healthcare providers to be alert for patients with illnesses associated with a rash. In working with the Washoe County Health District (WCHD), Renown is closely monitoring the spread of monkeypox in the community and looking to prevent and reduce the spread of monkeypox. To help to ease worries, we consulted with Paul De Leon, Infection Preventionist at Renown Health. What Exactly is Monkeypox? Monkeypox is a rare viral illness caused by the monkeypox virus — the same family of viruses that causes Smallpox. Although symptoms are similar to Smallpox, monkeypox symptoms are milder and rarely fatal. However, it's important to mention that this virus can be more severe for these susceptible groups: Immunocompromised Pregnant women A fetus or newborn baby Women who are breastfeeding Young children Those with severe skin diseases such as eczema How is Monkeypox Transmitted? The monkeypox virus is not easily transmitted but occurs through sustained person to person close contact with an infected individual. Monkeypox can also be transmitted through direct contact with infectious rash, scabs, or body fluids. Monkeypox can also be spread through prolonged intimate physical contact, such as kissing, cuddling or sex. Lastly, monkeypox can be spread through contaminated linens or bedding. Transmission through respiratory secretions is uncommon but has been reported after prolonged face-to-face contact with symptomatic individuals. In addition, pregnant women can spread the virus to their fetuses through the placenta. Monkeypox Testing If you think you have monkeypox, contact your primary care physician or other medical providers to obtain testing. Notify the provider ahead of time before entering the physical office. Signs & Symptoms This current outbreak of West African monkeypox does not have the typical presentation of classic monkeypox. Symptoms usually appear one to three weeks after infection and include: Pimple-like rash or blisters on the face, inside the mouth, and on other areas of the body, like the hands, feet, chest, genitals, or anus. The rash will go through serval stages, including scabs, before healing and may be painful or itchy. Other symptoms of monkeypox can include: Fever Headache Muscle aches and backache Swollen lymph nodes Chills Exhaustion Respiratory symptoms such as sore throat, nasal congestion, or cough Symptoms of monkeypox may occur before or after a rash with some individuals only report experience a rash. Individuals with monkeypox are infectious once symptoms begin and remain infectious until lesions form scabs, scabs fall off, and a fresh layer of skin forms. The illness typically lasts 2-4 weeks.
-
Celebrating Renown Health's Nursing Excellence Conference
Renown Health recently wrapped up the twentieth annual Nursing Excellence and Excellence in Critical Care Conferences, the conference theme was the Courageous Calling and over 400 nurses from specialty fields across the region attended to learn, reflect, build relationships and obtain continuing education units (CEUs). Celebrating The Courageous Calling During the first day of the Nursing Excellence Conference, local and national leaders presented topics including redefining resilience, documentation liability, transgender healthcare and caring for the homeless population. Among the list of impressive speakers were Chief Nurse Executive at Renown Health, Melodie Osborn, and Nora McInerny, writer and host of the "Terrible Thanks for Asking" podcast. On day two,speakers focused on the critical care specialties within nursing, including intensive care, emergency room, pediatric intensive care and neonatal intensive care. Topics covered included post-COVID-19 pulmonology with Dr. Graham, traumatic brain injuries with Dr. Demers, COVID-19 reflections with Anicia Beckwith, a discussion about "Mis C” with Dr. Healy, innovations in imaging with Dr. Rangaswamy and cardiology with Dr. Danaf. Thank you to our sponsors and raffle donors for making this event possible: Erik Olson and Larry Duncan, Jana Elliott, Melodie Osborn, Becky Haase, Lori Tuntland, Dr. Akbar, Dr. Lous, Mel Morris, Grand Sierra Resort, Renown Health Gift Shops, Renown Health Directors of Nursing, Renown Health Marketing & Communications Department, Renown Health Dermatology, Laser, & Skin Care and Renown Health Foundation. Learn more about finding purpose in the health of our community when working at Renown Health here.
Read More About Celebrating Renown Health's Nursing Excellence Conference
-
Two Years We Won't Forget: COVID-19 at Renown Health
On March 19, 2020, Renown Regional admitted the first patient in need of care while sick with COVID-19. Our providers navigated two years of a pandemic and overcame many challenges while providing the best care for our patients and the community. Anicia Beckwith’s series “The Art of Healing” captured Renown Health during this time. Let's take a look back on the last two years. February 2020: Standing Up the Hospital Incident Command System (HICS) On February 25, 2020, leaders at Renown Health stood up Renown’s Hospital Incident Command System (HICS), a standardized system used to organize response personnel and resources and manage response operations during emergencies and crises. March 2020: Temporary Deployable Medical Structure Placed Outside Renown Regional Emergency Department On March 12, 2020, Renown set up a deployable medical facility to serve as a respiratory illness screening center for emergency room patients at Renown Regional. A similar tent was also set up outside the emergency room at South Meadows Medical Center. This proactive measure helped our teams care for community members with respiratory illness symptoms while protecting patients and staff in the emergency department and other areas of the hospital. Check out photos of the tent here. Read the Reno Gazette Journal Article about the tent here. April 2020: Alternate Care Site at Mill Street Parking Structure at Renown Regional Renown’s HICS team decided to create an Alternate Care Site (ACS) in the Renown Regional Medical Center Mill Street parking structure. The ACS served additional hospitalized patients and allowed caregivers to remain on campus and still have access to existing hospital infrastructure such as lab, pharmacy, imaging, food services and other critical services. After just 10 days of construction, the ACS was completed on April 3, 2020 with space to hold up to 1,400 patients. Check out photos of the ACS under construction here. On Nov. 12, 2020, Renown opened the ACS to serve additional hospitalized patients diagnosed with COVID-19 who were clinically stable or improving. Healthcare workers at Renown cared for hundreds of patients at this site. In early Jan. 2021, the remaining patients returned home. Check out the video of Connie, a patient who received care in the ACS. April and July 2020: The LOVE Sculpture Placed at Renown Regional On April 16, 2020, during a time of darkness and uncertainty, Artown loaned Renown the LOVE sculpture, a one-ton aluminum piece of art created by artist Laura Kimpton and fabricated by Jeff Schomberg. The structure, which originally debuted at Burning Man, was lit up Renown Regional's main entrance on Mill St. Watch a video about the LOVE sculpture’s debut at Renown Regional. On July 13, 2020, thanks to the support of former board chair and community supporter Blake Smith and the Keyser Foundation, the LOVE sculpture is now a permanent fixture at Renown Health. Throughout the pandemic, it has served as a source of inspiration, hope and positivity for our community and care providers. Check out a video of the LOVE is Here to Stay celebration. June 2020: Renown Offers In-House COVID Testing In June 2020, the Renown laboratory team sprang into action to help meet the growing demand for COVID-19 testing amongst Washoe County residents and businesses. Renown invested in expanded staffing and in-house testing capabilities that ensured our teams could swab and process up to 1,000 COVID-19 tests for patients each day. All with results returning within hours. November 2020: Renown Introduces “Hospital At Home” Remote Monitoring In November 2020, six patients at Renown Regional Medical Center and Renown South Meadows Medical Center diagnosed with COVID-19 were outfitted with a remote Hospital at Home monitoring system. Renown clinicians plan to continue using this system to monitor upwards of 1,000 hospitalized patients and lower acuity patients from their homes. December 2020: Renown Administers COVID-19 Vaccines to Health Care Employees On Dec. 17, 2020, Renown began to vaccinate its healthcare workers. Among those receiving the first vaccines was Luis Martinez, a technician on Renown’s Clinical Decision Unit who cared for patients recovering from COVID-19 in the Alternate Care Site field hospital. Read the Reno Gazette Journal article about the COVID-19 vaccine rollout at Renown. January 2021: Renown Administers COVID-19 Vaccines to Community After several weeks of successful employee and volunteer drive-thru vaccination events, Renown supported the Washoe County Health District and the state in vaccinating Washoe County community members. Click here for a playlist of videos featuring Renown Health employees and patients talking about the importance of the COVID-19 vaccine. February 2021: Local Widow Inspires Renown to Change Visitor Supporter Policy Darlene Randolph’s husband Dave spent 17 days hospitalized at Renown Regional Medical Center before losing his battle with COVID-19 on December 13, 2020. Darlene wrote a passionate letter to Renown Health asking for the visitor policy that allowed patients with COVID-19 to receive visitors. In February 2021, Renown hospitals were among the first in the country to lift visitor restrictions for patients with COVID-19 to encourage families to be at the patient's bedside. Read Darlene’s full story here. May 2021: Renown Celebrates Volunteers, Partners and Community Who Aided in Vaccine Efforts In May 2021, Renown administered the last dose of COVID-19 vaccines to community members in Renown’s drive-thru clinic. Between January and May 2021, over 80,000 doses were administered at the drive-thru. View drone footage of this effort here. Click here to see pictures of vaccine volunteers and employees. November 2021: Renown Offers Vaccine for Children Ages 5+ In November 2021, Renown vaccinated children in the Reno-Sparks community with the 2-dose series in a limited round of weekend clinics. The vaccine clinics featured therapy dogs, local mascots and donuts donated by Doughboy’s Donuts. Click here to see pictures of the children’s vaccine clinics and watch a video about the clinics here.
Read More About Two Years We Won't Forget: COVID-19 at Renown Health
-
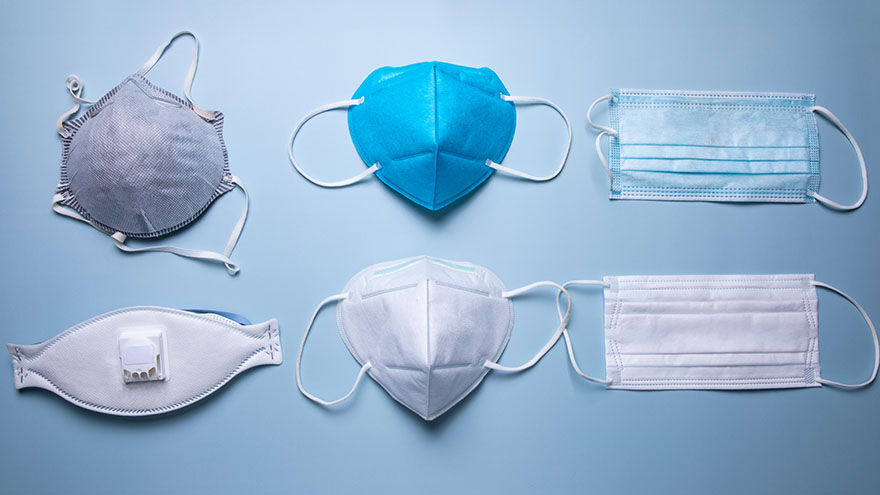
Avoid Counterfeits and Find the Right Protective Mask with This Helpful Guide
To better protect our patients, visitors and employees, cloth masks are no longer allowed at Renown facilities. Surgical masks, KN95 and N95 masks are allowed at Renown facilities. Appropriate face masks will be provided for visitors and patients who need one. With recent surges in the infectious COVID-19 omicron variant, many have sought to upgrade their face masks. But, let’s face it, shopping for face masks with adequate protection can be a challenge, especially considering the countless variations and the rise of counterfeit masks. Follow our straightforward guide below which includes some common red flags to help you discern between a high-quality face mask that provides proper protection and those that may be counterfeit. Types of Masks Qualities of a Real N95 Respirator Mask According to the National Institute for Occupational Safety and Health (NIOSH), N95 approved masks form a tight seal around your face and include a disposable respirator that removes particles including bacteria, viruses and dust as you breathe. N95 masks that are NIOSH approved undergo strict quality assurance and performance requirements to ensure mask respirators filter out up to 95% of hazardous particles. As a rule of thumb, N95 masks will not have ear loops, commonly used in cloth or surgical face masks. N95 respirators will contain two elastic bands or head straps that fasten behind the head, one securing the crown of the head and the other resting at the base of the neck, providing a snug fit and seal. Some other common signs that an N95 might be counterfeit include lack of all proper labeling, misspellings of NIOSH, decorative fabric and claiming to be approved for children. Currently, masks in adult sizes are the only masks to undergo NIOSH’s quality assurance and testing process. Respirators approved by NIOSH will include a testing certification (TC) approval number and will contain specific labeling on the facepiece of your mask. Find a full list of Center for Disease Control (CDC) and NIOSH requirements here. Identifying Real KN95 Respirator Masks Often preferred due to comfortability, the KN95 respirators were initially designed to meet Chinese standards for medical masks. Firstly, if a KN95 mask claims to be approved by the CDC, it is counterfeit as the CDC and NIOSH do not support any respiratory protective devices according to international standards. However, when KN95 masks are fitted and worn appropriately they do provide more protection than disposable masks. Legitimate KN95 masks will display a manufacturer number, GB2626-2019, ensuring accordance with current Chinese respirator standards for all masks made after July 1, 2021. Unlike N95 masks, it is important to note that KN95 masks are available in children's sizes. You might run into KN95 masks claiming to be “FDA (Food and Drug Administration) approved” or “FDA-registered,” but be aware that this does not mean much and is a misleading statement. What this indicates is that a mask maker has submitted paperwork to the FDA, but the product has not been thoroughly tested for proper filtration and protection. Surgical Masks Medical procedure masks often referred to as surgical or disposable masks vary in their protection according to a variety of factors including fit and filtration. The CDC defines medical procedure masks as “variably shaped, including flat pleated, cone-shaped, or duck-bill. Medical procedure masks are loose and are not expected to provide a reliable level of protection against airborne or aerosolized particles as N95 respirators regulated by the National Institute of for Occupational Safety and Health.” However, these types of masks provide more protection than cloth masks and are certainly better than wearing no mask at all. Often popular due to their level of comfort and cost-effectiveness, surgical masks can be knotted in the ear loop areas to provide a tighter seal and can be layered for additional filtration. Depending upon your budget and level of comfortability and protection, one variation of mask may suit you over another. Please remember to do your part in limiting the spread of COVID-19 and wear a surgical mask, KN95 or N95 mask when visiting Renown facilities.
Read More About Avoid Counterfeits and Find the Right Protective Mask with This Helpful Guide
-
COVID-19 Booster Shots, What You Need to Know
Getting the COVID-19 booster is the best way to protect yourself from severe illness or death due to COVID-19, and both the CDC and the FDA have approved booster shots for people ages 18 and older. So, with the holidays right around the corner and infection rates on the rise both in Nevada and nationally, the best thing you can do to prevent the continued spread of this deadly virus is to get boosted today. The Basics: Who: It is recommended that everyone 18 years or older get a COVID-19 booster shot. When: At least 6 months after completing your primary COVID-19 vaccination series. What: Any of the COVID-19 vaccines authorized in the United States. The CDC allows for mix and match dosing for booster shots. How: To make an appointment for your COVID-19 vaccine booster, please visit vaccines.gov today. Appointment Reminders: Don’t forget to bring your CDC vaccination record card to your appointment. Refresh yourself on the potential side effects and remember that these are normal signs your body is building up protection. Commonly Asked Questions: Q: Does anything change if I received the Johnson & Johnson as my first COVID-19 vaccine? A: If you received the Johnson & Johnson COVID-19 vaccine, you are elidable for a booster two months after completing your primary vaccine. Q: Is the formula the same for the boosters as it was for the primary vaccine? A: COVID-19 booster shots are the same formulation as the current COVID-19 vaccines. However, in the case of the Moderna COVID-19 vaccine booster shot, it is half the dose of the vaccine people get for their primary series. Q: Am I still considered “fully vaccinated” if I don’t receive a COVID-19 booster shot. A: Yes, everyone is still considered fully vaccinated two weeks after their second dose in a two-shot series, such as the Pfizer-BioNTech or Moderna vaccines, or two weeks after a single-dose vaccine, such as the J&J/Janssen vaccine. All information courtesy of the Center for Disease Control and Prevention. All information courtesy of the Center for Disease Control and Prevention
Read More About COVID-19 Booster Shots, What You Need to Know
-
Reno Widow Inspires New Visitor Policy for Renown
Renown Health is one of the country’s first health systems to lift visitor restrictions for patients with COVID-19 and encourage the family to be at the patient’s bedside. Read Darlene and Dave’s story to understand why we’re updating our visitor policy. Dave and Darlene Randolph found joy in exploring antique shops and garage sales to find damaged or discarded vintage pieces. Dave would spend many hours scraping, cleaning, sanding, and refinishing items, transforming them into functional, beautiful pieces of furniture. Every piece in their home rekindles a memory and has a story to tell. On Thanksgiving, when Dave was too ill to gather around their antique dining room table, Darlene called the ambulance. Ailing with COVID-19 for two weeks, Dave had not been improving. When the EMTs reached her home and asked Darlene what underlying conditions he had, she said, “all of them.” David was seriously ill. Hospitalized for COVID-19, their communications options were limited. The only way Darlene could communicate with Dave was on a video call or by telephone. Dave spent 17 days hospitalized at Renown Regional Medical Center in Reno. Darlene spent 17 days waiting by the phone for more information on his condition. Darlene said he had “up days and down days,” but thought he might be home, sitting at their antique dinner table for Christmas. Sadly, Dr. David Randolph lost his battle with COVID-19 on December 13, 2020, and died as he slept in a hospital bed. When Darlene wrote his obituary for the newspaper, she gave thanks to the “tremendous nurses and doctors at Renown Regional Medical Center, for providing his care during a time when the family could not be with him.” Taking Action to Inspire Change Darlene wished she could have been there. Over their 45-year marriage, she had always been there. Darlene said, “I had always been at his bedside, as his advocate, to help communicate and straighten things out.” As a registered dietician, she worked in hospitals, knew the protocol, and knew that Renown had a restricted visitor policy to stop the virus’s spread- to other patients, staff, and their family members. Still, she wished she could have spent more time with him. On Christmas Eve, she sat down and wrote to Renown leadership. “As the wife of a COVID patient who recently passed away in your hospital, I want to express my thanks to you and your staff for the care he received in the last days of his life. I am aware that the nurses and staff are working under dangerous conditions and risking their health and lives by caring for multiple COVID patients. The staff is gracious, concerned, and doing everything they can.” She continued, “I know procedures are changing every hour to try to stay ahead of this dangerous virus, and I am sharing my experiences, hoping they will be helpful when establishing policies that impact families.” Darlene explained that despite receiving assurances that Dave’s nurse or a doctor would call daily, sometimes they would forget. She explains in her letter, “how important it is, in these times when the family cannot visit, and has only infrequent communication and is anxiously waiting at home for information about their loved one, how much it means to get a call from someone caring for him at the hospital. If there is a way you can help assure nurses have time to make calls or assist patients in making calls because it is an important part of patient care.” A Person-Centered Visitor Policy After receiving her letter, Renown leadership called Mrs. David Randolph to thank her, offer his sympathies and ask if Renown could help in any way. Darlene asked if he might reconsider allowing families to visit hospital patients during treatment for COVID-19. As the COVID-19 situation has evolved, the policy has as well. Renown hospitals and medical practices now encourage limited visitors for all patients, including those diagnosed with COVID-19. Renown also has extra safety measures to protect the health of patients, visitors and healthcare employees. Darlene is very pleased that her letter inspired this shift in visitor policies for patients with COVID-19. She says, “I have always tried to think of ways I could help other families. Especially those senior couples where one has been hospitalized and the other is home. My wish is to help others.” Renown Health Visitor Policy Renown Health patients may identify two healthy adult “patient supporters” to accompany them on their hospital stay. For more details, visit our Patient Supporter Guidelines page.
Read More About Reno Widow Inspires New Visitor Policy for Renown
-
Pharmacists Answer Questions about the COVID-19 Vaccines
Vaccines that provide protection against the COVID-19 virus are bringing us closer to the end of this deadly pandemic. Two different COVID-19 vaccines are currently available in the U.S. today: one from Pfizer and the other from Moderna. Kate Ward, PharmD, BCPS, Director of Clinical Pharmacy at Renown Health and Adam Porath, PharmD, Vice President of Pharmacy at Renown, share what you need to know about these vaccines. When two COVID-19 vaccines were approved by the U.S. Food & Drug Administration (FDA) in December 2020, it was cause for celebration. Why? Because according to the CDC, the vaccines are 94 percent or more effective in providing protection against the COVID-19 virus! Many people are seeking information about the new Moderna and Pfizer vaccines. Below, our pharmacy leaders provide answers to some commonly asked questions. How do the COVID-19 Vaccines Work? The Pfizer and Moderna vaccines are both mRNA vaccines that help your immune system develop antibodies against the COVID-19 virus. The vaccines use messenger RNA, or mRNA, to show our bodies’ protein-making cells how to make the spike proteins of the COVID-19 virus. Our immune system reacts to these spike proteins by creating antibodies that can recognize and destroy them. So when a person is exposed to the virus in the future, they will be less likely to get sick. What are the Differences between the Pfizer and Moderna Vaccines? The Pfizer and Moderna COVID-19 vaccines are very similar, with just a few small differences worth noting. The main difference between the two vaccines is when you should receive your follow-up dose. Patients who receive a first dose of Pfizer should receive their second dose about three weeks later. Those who receive a first dose of Moderna should receive their follow-up vaccination roughly four weeks after their first dose. People 18 years and older can receive the Moderna vaccine while people 16 years and older can receive the Pfizer vaccine. Dosage for the Moderna vaccine is 0.5 ml (100 mcg). Dosage for the Pfizer vaccine is 0.3 ml (30 mcg).
Read More About Pharmacists Answer Questions about the COVID-19 Vaccines
-
COVID-19 Vaccine Expert Advice
With front-line workers receiving the first COVID-19 vaccinations, many of us are feeling hope, but also worry. As a result, we are joining with the Ad Council, the COVID Collaborative, HHS, CDC and NIAID (along with top health and medical organizations) to address your vaccine concerns and questions. Will the vaccine be available to everyone in Nevada? The Nevada Department of Health and Human Services (DHHS) is collaborating with health systems about the use of initially available, limited supplies of COVID-19 vaccines. They will provide guidance on the prioritization order of who will receive the vaccine. This will be based on available quantities, high-risk locations of work and certain other risk factors, and recommendations and guidance for public health agencies. The CDC has provided guidance to initially focus on the following groups: Healthcare personnel likely to be exposed to or treat people with COVID-19, nursing home residents and others in institutional settings; People at risk for severe illness from COVID-19 due to underlying medical conditions; People 65 years of age and older; Other essential workers. I worry the vaccine has been rushed The U.S. national vaccine safety system ensures that all vaccines are as safe as possible, and because vaccines are given to millions of healthy people to prevent serious diseases, they’re held to very high safety standards. COVID-19 vaccines are undergoing a rigorous development process that includes vaccinating tens of thousands of people who participate in a study to generate the needed clinical data. These clinical trials generate scientific data for the FDA to determine the safety and efficacy of each vaccine. It’s worth noting that the clinical studies to establish the safety and efficacy of the Covid-19 vaccines were as big and thorough as recent studies for other licensed vaccines (for example, the shingles vaccine). I'm concerned about the vaccine's side effects The most common side effects are very similar to those seen with most vaccines, such as: sore arms, fevers, and tiredness within 72 hours after the vaccine. These side effects usually mean that the vaccine is generating an immune response, indicating it is working. Short-term side effects observed in the leading COVID-19 vaccine trials include: Injection site pain and redness Fatigue Muscle aches and pains Joint pain Headache I’m afraid I’ll get COVID-19 from the vaccine None of the authorized and recommended COVID-19 vaccines, or COVID-19 vaccines currently in development in the United States, contain the live virus that causes COVID-19. This means that a COVID-19 vaccine cannot make you sick with COVID-19. Can children receive the COVID-19 vaccine? Not at the moment. In early clinical trials for various COVID-19 vaccines, only non-pregnant adults at least 18 years of age participated. However, clinical trials continue to expand those recruited to participate. The groups recommended to receive the vaccines could change in the future. As of now, it is recommended that children do not receive the vaccine. More information will be available from the vaccine manufacturers. I do not believe vaccines are effective Both this disease and the vaccine are new. We don’t know how long protection lasts for those who get infected or those who are vaccinated. What we do know is that COVID-19 has caused very serious illness and death for a lot of people. If you get COVID-19, you also risk giving it to loved ones who may get very sick. Getting a COVID-19 vaccine is a safer choice. The FDA is responsible for making sure that, just like any other medications, any FDA-authorized or approved COVID-19 vaccines are safe and they work. The EUA (Emergency Use Authorization) will not be provided if the FDA feels that the vaccine is unsafe. I can't get vaccines to due to a medical condition Adults of any age with certain underlying medical conditions are at increased risk for severe illness from the virus that causes COVID-19. mRNA COVID-19 vaccines may be administered to people with underlying medical conditions provided they have not had a severe allergic reaction to any of the ingredients in the vaccine. The following information aims to help people in the groups listed below make an informed decision about receiving the mRNA COVID-19 vaccine. It is extremely important to speak with your doctor regarding your specific medical condition, and always follow their strict advice regarding the COVID-19 vaccine, or any other vaccines. Sources: Renown COVID-19 Ad Council COVID Collaborative U.S. Department of Health & Human Services Centers for Disease Control and Prevention National Institute of Allergy and Infectious Disease