Search
-
Researchers at Renown Seek Convalescent Plasma Study Participants
Physician researchers seek to understand how the immune system responds to COVID-19 and create a healthier Nevada. During the early stages of the pandemic, convalescent plasma was considered the only viable treatment option available for patients with COVID-19. Convalescent plasma is the component of the blood from recovered patients that may contain COVID-19 antibodies that help fight the infection. The National Institutes of Health has since developed treatment guidelines for COVID-19 based on clinical trial data and many studies are still underway worldwide assessing various additional treatment options. Convalescent plasma was in high demand but difficult to locate for COVID-19 patients in the northern Nevada area. A 24-year-old nursing assistant, Austin Meegan, was hospitalized and spent weeks staving off kidney and lung failure before learning he was eligible for an experimental blood transfusion that showed promise in treating COVID-19. Doctors estimated Meegan had only about a 3% chance of tracking down a donor to match his rare blood type. A COVID-19 survivor, Thomas Gibson, a Texas resident with the same blood type, traveled to Reno to donate his viral antibodies and a convalescent plasma donation credited with helping to save Meegan’s life. Physician clinical researchers and scientists at Renown and the University of Nevada, Reno School of Medicine (UNR Med) knew they needed to create better options for patients and physicians. Clinical researchers developed a study to help other patients like Austin, and pleaded for donations from recovered COVID-19 patients to donate their convalescent plasma. The researcher teams looked to understand how the body’s immune system responds to the virus over time, to aid them in developing new treatments for COVID-19. “The world’s capacity to get through the COVID crisis will depend on four things — science, technology, innovation and partnerships, says Tony Slonim, MD, DrPH, President and CEO of Renown Health. “Taking lab bench discoveries to the bedside of patients in an efficacious and timely manner is not easy, but with UNR Med and our partners, we are making great strides in advancing clinical research which has the power to save lives and to create a healthier Nevada.” “It’s tremendously promising to partner on clinical research that will not only help us better understand the disease, but help inform treatment for those combatting COVID-19 that has had such a devastating impact on Nevadans, our nation and the world,” says UNR Med Dean Thomas L. Schwenk, MD. Renown, UNR Med and other area health care partners collaborated with Vitalant to collect plasma from recovered donors for a study on the treatment's efficacy. Eligible donors are at least 18 years old, weigh more than 110 pounds and are healthy. Donors had fully recovered from a confirmed diagnosis of COVID-19. Project coordinators at the Renown Research Office were overwhelmed by the community’s support and plasma blood donations. Additional partnerships with the Washoe County Health District, the State of Nevada and the Governor’s office, Saint Mary’s Medical Center, Northern Nevada Medical Center, Carson Tahoe Health and the VA Sierra Nevada Health Care System, along with many area health care providers helped the team meet their goals of enrolling 120 eligible participants in the study. “Our success in this study rests heavily on the support of our great community, as well as the innovation and collaboration demonstrated by Renown and UNR Med,” said Sara Healy, MD, MPH, principal investigator of the study and a pediatric infectious disease physician at Renown Children’s Hospital and UNR Med. “We are proud to be at the forefront of conducting essential research during such a pivotal time in history, and look forward to our continued partnership as we continue this important work.” “The control of COVID-19 in our communities relies on testing. The study that is being launched to develop a sensitive, specific and easier way to collect specimens (blood) is advancing the field and brings promise towards getting to our common goal of having the right diagnostic test for the right clinical situation at the right time,” says Mark Riddle, MD, DrPH, FISTM, associate investigator of the study and associate dean for clinical research and professor at UNR Med's Department of Internal Medicine and Medical Research. The research team is now asking area residents to participate in a study to analyze the efficacy of two COVID-19 tests. Participants will undergo two blood tests: one being a finger stick to provide results for a rapid test, and the other is a traditional venipuncture draw confirming the presence or absence of COVID-19 antibodies. This study is a collaboration with InBios International, Inc., a leading biotechnology company based in Seattle. Researchers are seeking: Individuals who have confirmed positive for COVID-19 and who have recently recovered from the virus. Study participants must be within 7-28 days from the onset of their symptoms. Individuals who have recently tested negative for COVID-19 and have never tested positive. Those who are interested in participating in the study may contact project coordinators at the Renown Research Office at (775) 982-3646, or via e-mail at covidplasmascreening@renown.org, 7:30 A.M. – 5:00 P.M., Monday through Friday. Individuals aged 18-75 in general good health are encouraged to consider participating in this ongoing study. There is no cost to participate in this study and participation is voluntary. An individual’s decision to participate will not affect their current or future relations with their health care provider(s), health district, or the community. Those who decide to participate are free to withdraw at any time. “Time is of the essence with COVID. If we can get test results to people and their clinicians in a more timely way, we can make a faster diagnosis of a patient's condition, says Christopher M. Kozlowski, MD, MHA, Renown's institutional research officer and Medical Director/VP of Renown Institute for Heart & Vascular Health. “As we refine the accuracy of our testing; we are applying sensitivity and specificity testing for true negative and true positive results. This provides people with more timely and accurate results and better quality care.” About Renown Health Renown Health is a locally governed, not-for-profit integrated healthcare network serving northern Nevada, Lake Tahoe and northeast California. Renown is one of the region’s largest private employers with a workforce of more than 7,000. It comprises three acute care hospitals, a rehabilitation hospital, the area’s most comprehensive medical group and urgent care network, and the region’s largest and only locally owned not-for-profit insurance company, Hometown Health. Renown has a long tradition and commitment to improve the care and the health of our community. About the University of Nevada, Reno School of Medicine The University of Nevada, Reno School of Medicine (UNR Med), Nevada’s first medical school, is a community-based, research-intensive medical school with a statewide vision for a healthy Nevada. Established in 1969, UNR Med is improving the health and well-being of all Nevadans and their communities through excellence in student education, postgraduate training and clinical care, research with local, national and global impact and a culture of diversity and inclusion. For more information, visit med.unr.edu.
Read More About Researchers at Renown Seek Convalescent Plasma Study Participants
-
Palliative and Supportive Care
Compassionate Care Palliative and Supportive Care provides specialized medical care for serious illnesses and diseases, including advanced kidney failure or heart disease. Palliative Care helps to: Prevent and relieve suffering to help build the best possible quality of life. Add value to standard therapies by assisting with advanced illness planning and symptom management. Palliative & Supportive Care may be needed if: You've had multiple hospital admissions for severe illness. Severe pain, nausea, fatigue or other symptoms impacting quality of life and you are reconsidering treatment plans. Treatments are no longer working. You're feeling hapless or discouraged about the future due to your serious illness. Talk to your doctor to find out if palliative care is the right choice for you or your family member. Your Care Team Your palliative care support team comprises doctors, nurses, chaplains, social workers and other specialists who work together with you to provide extra support. Your care team can help: Facilitate close communication between you and your physician team, as well as nurses and specialists. Offer medical assessments and symptom management to help reduce pain, nausea, fatigue, shortness of breath and anxiety. Improve the ability to tolerate medical treatments and fain the strength to live a productive daily life. Explain treatment options and the decision making process regarding care. Navigate the healthcare process. Lend emotional and spiritual support.
-
Hospice Care
When medical treatments no longer offer a cure, Hospice Care offers a special way to care for you and your family who are faced with a life-limiting illness. Serving Washoe, Lyon, Storey and Carson Counties, our team is available 24 hours a day, seven days a week. Hospice staff receive special training to care for all types of physical and emotional symptoms that cause pain, discomfort and distress. When considering your options for end-of-life transition, our team is available to answer questions and discuss if Renown Hospice Care will meet your needs. Your hospice care team includes Medical Director Registered Nurses Certified Nursing Aides Medical Social Workers Chaplains Registered Dietitians Trained Volunteers
-
Helpful Caregivers Make a Wedding Dream Come True
A wedding is a big day for the wedding couple, but it’s also special for loved ones. A patient at Renown, Ken, got to take part in his daughter’s special day as her wedding plans changed to accommodate his medical condition. Grab some tissues and read how Renown’s team of compassionate caregivers and chaplains planned a wedding in Fianna’s Healing Garden. Ken was hospitalized at Renown Regional Medical Center where he was battling a lung problem – which was unrelated to COVID-19 – and his condition worsened rapidly on Wednesday, Aug. 12. His family made the decision to transition him to palliative care, which helps patients near the end of their lives remain comfortable, while supporting their dignity and quality of life. Ken’s medical condition altered wedding plans for his daughter, Chandra, and her fiancé, Tyler, who were planning to tie the knot later in 2020. Chandra wanted her father there, but knew he could not leave the hospital. That’s why Chandra’s sister, Heather, approached Ken’s care team with a request to have a small wedding ceremony at the hospital. Planning the Wedding A member of Ken’s care team, Amy Heston, registered nurse (RN), began planning how the wedding could be held outdoors in Fianna's Healing Garden in the E. L. Wiegand Pavilion, which was donated by the E. L. Wiegand Foundation. In 24 hours, Amy planned a wedding ceremony with the help of her colleague, Breyanna Aufiero, RN; the Renown Spiritual Care team; and nursing leaders on the coronary intensive care unit (ICU). Together, they decorated the aisle in the garden with flowers and battery-operated candles. They also made a sign for Ken’s hospital bed, which read, “Father of the Bride,” and crafted a bow tie for him to wear for the special occasion. With visitor restrictions in place at the hospital due to coronavirus (COVID-19), having the wedding outside in the Healing Garden allowed for more members of Ken’s family to attend including his wife, Charlotte, and his dog, Bella. Every step in planning the wedding required thoughtful and thorough care coordination so Ken could participate. His breathing was supported by oxygen and special arrangements were made to transport the oxygen tanks he needed to take part in his daughter’s wedding. Amy worked with respiratory technician, Kasey Benfield, and critical care technician, Ruben Duckworth, to ensure Ken’s oxygen needs were met using portable machines. Celebrating Love and Life Together Ken’s team of caregivers bathed him and shaved his face so he could look and feel his best for the ceremony. They put on his bow tie, covered his bed in decorations and his favorite blue, flannel blanket, and wheeled his bed outside for the ceremony. Renown associate chaplains Terri Domitrovich and Susan Palwick coordinated music and performed the ceremony for Chandra and Tyler on Thursday, Aug. 13, 2020. The bride and groom shared their first dance in the garden and Ken’s care team provided water and treats to give the family a full wedding experience. Shortly after the ceremony, Ken passed away. This wedding provided Ken and his family meaningful memories for their big life-changing moments as they celebrated and said goodbye. “Seeing Ken surrounded by family he never would have gotten to see again while in the hospital, watching him get to share a father-daughter dance with Chandra on her wedding day, and having him tell me that this day meant more to them than we would ever know were some of the most moving moments I’ve witnessed as a nurse,” Amy said. “I am so thankful for the team we have here. I know that this beautiful day wouldn’t have happened without the help of every single person who gave their time, money, creativity and passion to make it a day to remember.”
Read More About Helpful Caregivers Make a Wedding Dream Come True
-
COVID-19 Booster Shots, What You Need to Know
Getting the COVID-19 booster is the best way to protect yourself from severe illness or death due to COVID-19, and both the CDC and the FDA have approved booster shots for people ages 18 and older. So, with the holidays right around the corner and infection rates on the rise both in Nevada and nationally, the best thing you can do to prevent the continued spread of this deadly virus is to get boosted today. The Basics: Who: It is recommended that everyone 18 years or older get a COVID-19 booster shot. When: At least 6 months after completing your primary COVID-19 vaccination series. What: Any of the COVID-19 vaccines authorized in the United States. The CDC allows for mix and match dosing for booster shots. How: To make an appointment for your COVID-19 vaccine booster, please visit vaccines.gov today. Appointment Reminders: Don’t forget to bring your CDC vaccination record card to your appointment. Refresh yourself on the potential side effects and remember that these are normal signs your body is building up protection. Commonly Asked Questions: Q: Does anything change if I received the Johnson & Johnson as my first COVID-19 vaccine? A: If you received the Johnson & Johnson COVID-19 vaccine, you are elidable for a booster two months after completing your primary vaccine. Q: Is the formula the same for the boosters as it was for the primary vaccine? A: COVID-19 booster shots are the same formulation as the current COVID-19 vaccines. However, in the case of the Moderna COVID-19 vaccine booster shot, it is half the dose of the vaccine people get for their primary series. Q: Am I still considered “fully vaccinated” if I don’t receive a COVID-19 booster shot. A: Yes, everyone is still considered fully vaccinated two weeks after their second dose in a two-shot series, such as the Pfizer-BioNTech or Moderna vaccines, or two weeks after a single-dose vaccine, such as the J&J/Janssen vaccine. All information courtesy of the Center for Disease Control and Prevention. All information courtesy of the Center for Disease Control and Prevention
Read More About COVID-19 Booster Shots, What You Need to Know
-
Reno Widow Inspires New Visitor Policy for Renown
Renown Health is one of the country’s first health systems to lift visitor restrictions for patients with COVID-19 and encourage the family to be at the patient’s bedside. Read Darlene and Dave’s story to understand why we’re updating our visitor policy. Dave and Darlene Randolph found joy in exploring antique shops and garage sales to find damaged or discarded vintage pieces. Dave would spend many hours scraping, cleaning, sanding, and refinishing items, transforming them into functional, beautiful pieces of furniture. Every piece in their home rekindles a memory and has a story to tell. On Thanksgiving, when Dave was too ill to gather around their antique dining room table, Darlene called the ambulance. Ailing with COVID-19 for two weeks, Dave had not been improving. When the EMTs reached her home and asked Darlene what underlying conditions he had, she said, “all of them.” David was seriously ill. Hospitalized for COVID-19, their communications options were limited. The only way Darlene could communicate with Dave was on a video call or by telephone. Dave spent 17 days hospitalized at Renown Regional Medical Center in Reno. Darlene spent 17 days waiting by the phone for more information on his condition. Darlene said he had “up days and down days,” but thought he might be home, sitting at their antique dinner table for Christmas. Sadly, Dr. David Randolph lost his battle with COVID-19 on December 13, 2020, and died as he slept in a hospital bed. When Darlene wrote his obituary for the newspaper, she gave thanks to the “tremendous nurses and doctors at Renown Regional Medical Center, for providing his care during a time when the family could not be with him.” Taking Action to Inspire Change Darlene wished she could have been there. Over their 45-year marriage, she had always been there. Darlene said, “I had always been at his bedside, as his advocate, to help communicate and straighten things out.” As a registered dietician, she worked in hospitals, knew the protocol, and knew that Renown had a restricted visitor policy to stop the virus’s spread- to other patients, staff, and their family members. Still, she wished she could have spent more time with him. On Christmas Eve, she sat down and wrote to Renown leadership. “As the wife of a COVID patient who recently passed away in your hospital, I want to express my thanks to you and your staff for the care he received in the last days of his life. I am aware that the nurses and staff are working under dangerous conditions and risking their health and lives by caring for multiple COVID patients. The staff is gracious, concerned, and doing everything they can.” She continued, “I know procedures are changing every hour to try to stay ahead of this dangerous virus, and I am sharing my experiences, hoping they will be helpful when establishing policies that impact families.” Darlene explained that despite receiving assurances that Dave’s nurse or a doctor would call daily, sometimes they would forget. She explains in her letter, “how important it is, in these times when the family cannot visit, and has only infrequent communication and is anxiously waiting at home for information about their loved one, how much it means to get a call from someone caring for him at the hospital. If there is a way you can help assure nurses have time to make calls or assist patients in making calls because it is an important part of patient care.” A Person-Centered Visitor Policy After receiving her letter, Renown leadership called Mrs. David Randolph to thank her, offer his sympathies and ask if Renown could help in any way. Darlene asked if he might reconsider allowing families to visit hospital patients during treatment for COVID-19. As the COVID-19 situation has evolved, the policy has as well. Renown hospitals and medical practices now encourage limited visitors for all patients, including those diagnosed with COVID-19. Renown also has extra safety measures to protect the health of patients, visitors and healthcare employees. Darlene is very pleased that her letter inspired this shift in visitor policies for patients with COVID-19. She says, “I have always tried to think of ways I could help other families. Especially those senior couples where one has been hospitalized and the other is home. My wish is to help others.” Renown Health Visitor Policy Renown Health patients may identify two healthy adult “patient supporters” to accompany them on their hospital stay. For more details, visit our Patient Supporter Guidelines page.
Read More About Reno Widow Inspires New Visitor Policy for Renown
-
Pharmacists Answer Questions about the COVID-19 Vaccines
Vaccines that provide protection against the COVID-19 virus are bringing us closer to the end of this deadly pandemic. Two different COVID-19 vaccines are currently available in the U.S. today: one from Pfizer and the other from Moderna. Kate Ward, PharmD, BCPS, Director of Clinical Pharmacy at Renown Health and Adam Porath, PharmD, Vice President of Pharmacy at Renown, share what you need to know about these vaccines. When two COVID-19 vaccines were approved by the U.S. Food & Drug Administration (FDA) in December 2020, it was cause for celebration. Why? Because according to the CDC, the vaccines are 94 percent or more effective in providing protection against the COVID-19 virus! Many people are seeking information about the new Moderna and Pfizer vaccines. Below, our pharmacy leaders provide answers to some commonly asked questions. How do the COVID-19 Vaccines Work? The Pfizer and Moderna vaccines are both mRNA vaccines that help your immune system develop antibodies against the COVID-19 virus. The vaccines use messenger RNA, or mRNA, to show our bodies’ protein-making cells how to make the spike proteins of the COVID-19 virus. Our immune system reacts to these spike proteins by creating antibodies that can recognize and destroy them. So when a person is exposed to the virus in the future, they will be less likely to get sick. What are the Differences between the Pfizer and Moderna Vaccines? The Pfizer and Moderna COVID-19 vaccines are very similar, with just a few small differences worth noting. The main difference between the two vaccines is when you should receive your follow-up dose. Patients who receive a first dose of Pfizer should receive their second dose about three weeks later. Those who receive a first dose of Moderna should receive their follow-up vaccination roughly four weeks after their first dose. People 18 years and older can receive the Moderna vaccine while people 16 years and older can receive the Pfizer vaccine. Dosage for the Moderna vaccine is 0.5 ml (100 mcg). Dosage for the Pfizer vaccine is 0.3 ml (30 mcg).
Read More About Pharmacists Answer Questions about the COVID-19 Vaccines
-
COVID-19 Vaccine Expert Advice
With front-line workers receiving the first COVID-19 vaccinations, many of us are feeling hope, but also worry. As a result, we are joining with the Ad Council, the COVID Collaborative, HHS, CDC and NIAID (along with top health and medical organizations) to address your vaccine concerns and questions. Will the vaccine be available to everyone in Nevada? The Nevada Department of Health and Human Services (DHHS) is collaborating with health systems about the use of initially available, limited supplies of COVID-19 vaccines. They will provide guidance on the prioritization order of who will receive the vaccine. This will be based on available quantities, high-risk locations of work and certain other risk factors, and recommendations and guidance for public health agencies. The CDC has provided guidance to initially focus on the following groups: Healthcare personnel likely to be exposed to or treat people with COVID-19, nursing home residents and others in institutional settings; People at risk for severe illness from COVID-19 due to underlying medical conditions; People 65 years of age and older; Other essential workers. I worry the vaccine has been rushed The U.S. national vaccine safety system ensures that all vaccines are as safe as possible, and because vaccines are given to millions of healthy people to prevent serious diseases, they’re held to very high safety standards. COVID-19 vaccines are undergoing a rigorous development process that includes vaccinating tens of thousands of people who participate in a study to generate the needed clinical data. These clinical trials generate scientific data for the FDA to determine the safety and efficacy of each vaccine. It’s worth noting that the clinical studies to establish the safety and efficacy of the Covid-19 vaccines were as big and thorough as recent studies for other licensed vaccines (for example, the shingles vaccine). I'm concerned about the vaccine's side effects The most common side effects are very similar to those seen with most vaccines, such as: sore arms, fevers, and tiredness within 72 hours after the vaccine. These side effects usually mean that the vaccine is generating an immune response, indicating it is working. Short-term side effects observed in the leading COVID-19 vaccine trials include: Injection site pain and redness Fatigue Muscle aches and pains Joint pain Headache I’m afraid I’ll get COVID-19 from the vaccine None of the authorized and recommended COVID-19 vaccines, or COVID-19 vaccines currently in development in the United States, contain the live virus that causes COVID-19. This means that a COVID-19 vaccine cannot make you sick with COVID-19. Can children receive the COVID-19 vaccine? Not at the moment. In early clinical trials for various COVID-19 vaccines, only non-pregnant adults at least 18 years of age participated. However, clinical trials continue to expand those recruited to participate. The groups recommended to receive the vaccines could change in the future. As of now, it is recommended that children do not receive the vaccine. More information will be available from the vaccine manufacturers. I do not believe vaccines are effective Both this disease and the vaccine are new. We don’t know how long protection lasts for those who get infected or those who are vaccinated. What we do know is that COVID-19 has caused very serious illness and death for a lot of people. If you get COVID-19, you also risk giving it to loved ones who may get very sick. Getting a COVID-19 vaccine is a safer choice. The FDA is responsible for making sure that, just like any other medications, any FDA-authorized or approved COVID-19 vaccines are safe and they work. The EUA (Emergency Use Authorization) will not be provided if the FDA feels that the vaccine is unsafe. I can't get vaccines to due to a medical condition Adults of any age with certain underlying medical conditions are at increased risk for severe illness from the virus that causes COVID-19. mRNA COVID-19 vaccines may be administered to people with underlying medical conditions provided they have not had a severe allergic reaction to any of the ingredients in the vaccine. The following information aims to help people in the groups listed below make an informed decision about receiving the mRNA COVID-19 vaccine. It is extremely important to speak with your doctor regarding your specific medical condition, and always follow their strict advice regarding the COVID-19 vaccine, or any other vaccines. Sources: Renown COVID-19 Ad Council COVID Collaborative U.S. Department of Health & Human Services Centers for Disease Control and Prevention National Institute of Allergy and Infectious Disease
-
Monkeypox: A Renown Expert Weighs In
Renown Health is closely following the national outbreak of the monkeypox virus and urging healthcare providers to be alert for patients with illnesses associated with a rash. In working with the Washoe County Health District (WCHD), Renown is closely monitoring the spread of monkeypox in the community and looking to prevent and reduce the spread of monkeypox. To help to ease worries, we consulted with Paul De Leon, Infection Preventionist at Renown Health. What Exactly is Monkeypox? Monkeypox is a rare viral illness caused by the monkeypox virus — the same family of viruses that causes Smallpox. Although symptoms are similar to Smallpox, monkeypox symptoms are milder and rarely fatal. However, it's important to mention that this virus can be more severe for these susceptible groups: Immunocompromised Pregnant women A fetus or newborn baby Women who are breastfeeding Young children Those with severe skin diseases such as eczema How is Monkeypox Transmitted? The monkeypox virus is not easily transmitted but occurs through sustained person to person close contact with an infected individual. Monkeypox can also be transmitted through direct contact with infectious rash, scabs, or body fluids. Monkeypox can also be spread through prolonged intimate physical contact, such as kissing, cuddling or sex. Lastly, monkeypox can be spread through contaminated linens or bedding. Transmission through respiratory secretions is uncommon but has been reported after prolonged face-to-face contact with symptomatic individuals. In addition, pregnant women can spread the virus to their fetuses through the placenta. Monkeypox Testing If you think you have monkeypox, contact your primary care physician or other medical providers to obtain testing. Notify the provider ahead of time before entering the physical office. Signs & Symptoms This current outbreak of West African monkeypox does not have the typical presentation of classic monkeypox. Symptoms usually appear one to three weeks after infection and include: Pimple-like rash or blisters on the face, inside the mouth, and on other areas of the body, like the hands, feet, chest, genitals, or anus. The rash will go through serval stages, including scabs, before healing and may be painful or itchy. Other symptoms of monkeypox can include: Fever Headache Muscle aches and backache Swollen lymph nodes Chills Exhaustion Respiratory symptoms such as sore throat, nasal congestion, or cough Symptoms of monkeypox may occur before or after a rash with some individuals only report experience a rash. Individuals with monkeypox are infectious once symptoms begin and remain infectious until lesions form scabs, scabs fall off, and a fresh layer of skin forms. The illness typically lasts 2-4 weeks.
-
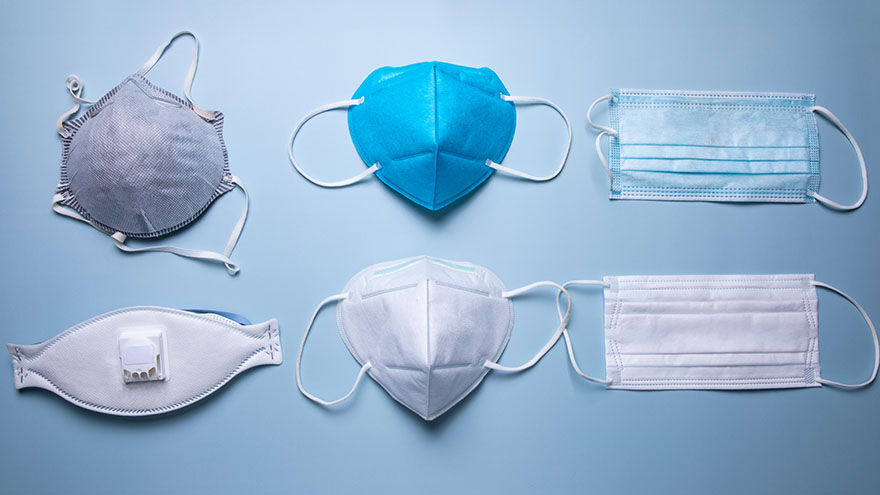
Avoid Counterfeits and Find the Right Protective Mask with This Helpful Guide
To better protect our patients, visitors and employees, cloth masks are no longer allowed at Renown facilities. Surgical masks, KN95 and N95 masks are allowed at Renown facilities. Appropriate face masks will be provided for visitors and patients who need one. With recent surges in the infectious COVID-19 omicron variant, many have sought to upgrade their face masks. But, let’s face it, shopping for face masks with adequate protection can be a challenge, especially considering the countless variations and the rise of counterfeit masks. Follow our straightforward guide below which includes some common red flags to help you discern between a high-quality face mask that provides proper protection and those that may be counterfeit. Types of Masks Qualities of a Real N95 Respirator Mask According to the National Institute for Occupational Safety and Health (NIOSH), N95 approved masks form a tight seal around your face and include a disposable respirator that removes particles including bacteria, viruses and dust as you breathe. N95 masks that are NIOSH approved undergo strict quality assurance and performance requirements to ensure mask respirators filter out up to 95% of hazardous particles. As a rule of thumb, N95 masks will not have ear loops, commonly used in cloth or surgical face masks. N95 respirators will contain two elastic bands or head straps that fasten behind the head, one securing the crown of the head and the other resting at the base of the neck, providing a snug fit and seal. Some other common signs that an N95 might be counterfeit include lack of all proper labeling, misspellings of NIOSH, decorative fabric and claiming to be approved for children. Currently, masks in adult sizes are the only masks to undergo NIOSH’s quality assurance and testing process. Respirators approved by NIOSH will include a testing certification (TC) approval number and will contain specific labeling on the facepiece of your mask. Find a full list of Center for Disease Control (CDC) and NIOSH requirements here. Identifying Real KN95 Respirator Masks Often preferred due to comfortability, the KN95 respirators were initially designed to meet Chinese standards for medical masks. Firstly, if a KN95 mask claims to be approved by the CDC, it is counterfeit as the CDC and NIOSH do not support any respiratory protective devices according to international standards. However, when KN95 masks are fitted and worn appropriately they do provide more protection than disposable masks. Legitimate KN95 masks will display a manufacturer number, GB2626-2019, ensuring accordance with current Chinese respirator standards for all masks made after July 1, 2021. Unlike N95 masks, it is important to note that KN95 masks are available in children's sizes. You might run into KN95 masks claiming to be “FDA (Food and Drug Administration) approved” or “FDA-registered,” but be aware that this does not mean much and is a misleading statement. What this indicates is that a mask maker has submitted paperwork to the FDA, but the product has not been thoroughly tested for proper filtration and protection. Surgical Masks Medical procedure masks often referred to as surgical or disposable masks vary in their protection according to a variety of factors including fit and filtration. The CDC defines medical procedure masks as “variably shaped, including flat pleated, cone-shaped, or duck-bill. Medical procedure masks are loose and are not expected to provide a reliable level of protection against airborne or aerosolized particles as N95 respirators regulated by the National Institute of for Occupational Safety and Health.” However, these types of masks provide more protection than cloth masks and are certainly better than wearing no mask at all. Often popular due to their level of comfort and cost-effectiveness, surgical masks can be knotted in the ear loop areas to provide a tighter seal and can be layered for additional filtration. Depending upon your budget and level of comfortability and protection, one variation of mask may suit you over another. Please remember to do your part in limiting the spread of COVID-19 and wear a surgical mask, KN95 or N95 mask when visiting Renown facilities.
Read More About Avoid Counterfeits and Find the Right Protective Mask with This Helpful Guide