Search
Results for 'medical records'
Clear-
Department Spotlight: Pharmacy
When it comes to each patient’s healthcare journey, medication is often a key chapter. After all, medication is one of the most common treatment methods to help patients on the road back to health. In 2023, 4.83 billion prescriptions were filled in the U.S., and with this number only anticipated to rise annually, having an expert pharmacy team on your side to make certain you are prescribed the ideal dosage to treat your condition, prepare your prescriptions on time and help you manage your medications responsibly is important. Fortunately, at Renown Health, we have best-in-class inpatient and outpatient pharmacy teams to fill both prescriptions and promises for excellent care. Renown Pharmacy plays a vital role in helping us foster a health system that prioritizes patient well-being above all else. This department exemplifies the impact that a unified, expert pharmacy team can have on patient outcomes now and in the future. The Masters of Medication Spanning three hospitals plus ambulatory locations across the health system, Renown’s growing pharmacy team – full of dedicated pharmacists, pharmacy technicians and even medical assistants – manages medications in a wide variety of patient settings, touching nearly every aspect of the healthcare continuum: Outpatient Retail Pharmacies Renown Regional Medical Center – 75 Pringle Way The Healthcare Center – 21 Locust Street Renown South Meadows Medical Center – 10101 Double R Blvd Inpatient Pharmacies Renown Rehabilitation Hospital Renown Regional Medical Center (including Renown Children’s Hospital) Renown South Meadows Medical Center COMING SOON: Conrad Breast Center Pharmacy (in honor of Kristina Ferrari) in the Specialty Care Center at Renown South Meadows Ambulatory Pharmacies Anticoagulation Services – Institute for Heart & Vascular Health (IHVH) Pharmacotherapy Program – IHVH and Renown Medical Group Locations Congestive Heart Failure Pharmacotherapy Program – Center for Advanced Medicine B at Renown Regional Chronic Obstructive Pulmonary Disease (COPD) Pharmacotherapy Program – Renown South Meadows Endocrinology Pharmacotherapy Program – Renown South Meadows Additional Pharmacy Programs Medical Reconciliation Pharmacy Residency Clinical pharmacists at Renown bridge the gap between medicine and compassionate support, making sure each patient receives personalized care one prescription at a time. “There are various roles pharmacists play within Renown,” said Clarissa Munoz, Clinical Pharmacist in the Renown Regional Inpatient Pharmacy. “Staff pharmacists work diligently to ensure correct medications are dispensed, and if compounded, make sure they were prepared properly. They also work hard to answer medication messages and phone calls, help verify orders and make sure ode trays/RSI kits are appropriately stocked and ready when needed. Clinical pharmacists work from satellite pharmacies on the floor and focus on reviewing patient charts and aim to provide additional interventions to the providers to optimize treatment strategies. We also serve as a resource for nursing staff and help answer medication questions.” “My role in the pharmacy is pretty expansive,” added Chanelle Ajimura, Clinical Pharmacist in the Renown Regional Outpatient Pharmacy. “I maintain inventory to confirm patients can receive their medications in a timely manner both for our discharge and retail patients while balancing the Meds to Beds program, which offers medication delivery to the bedside and bedside medication counseling; collaborating with an interdisciplinary team to find the most affordable price for patients; and verifying that the dose, strength, indication, etc. is appropriate for the patient from start to finish.” “In the pharmacy, I make sure patients are receiving appropriate drug therapy by checking for major drug interactions and ensuring appropriate dosing,” added Courtney Church, Clinical Pharmacist in the Renown Regional Outpatient Pharmacy. “I also make recommendations to providers so patients can get cost-effective therapy.” Our pharmacy technicians work behind-the-scenes ensuring efficient medication management, making a difference in the lives of patients every day. “A pharmacy technician is responsible for making sure the patient gets their medications on time and at the lowest price possible,” said Nate Graham, Pharmacy Technician in the Renown Regional Outpatient Pharmacy. “This is done by working with patients, insurance companies and case workers. We fill prescriptions, enter prescriptions into our system, receive and send orders for medications and maintain a clean pharmacy with an accurate inventory.” “We do a variety of things; the task people probably know the most is counting out the medications and putting them in the amber vials,” added Rachel Vallin, Pharmacy Technician in the Renown Regional Outpatient Pharmacy. “We also help patients at the front of the pharmacy, ring out their prescriptions, answer some basic questions (deferring to a pharmacist as necessary) and billing insurance. Meds to Beds is my favorite part because I feel the most involved. I take medications to patients who are discharging up to their hospital rooms so they have it with them when they leave.” “As a technician, I confirm that all medications of new admissions are available in our machines prior to admitting and then maintain stock during each patient’s stay,” added Tammara Axtman, Pharmacy Technician at Renown Rehabilitation Hospital. "I also assist our nurses when needed in regard to any of their questions with both EPIC and Omnicell.” Our pharmacy team is also on the move all across our health system, thanks to our Ambulatory Pharmacy programs. For patients experiencing a serious heart, lung, or endocrine condition that requires ongoing drug therapy maintenance and guidance, our ambulatory pharmacies step in to carefully monitor how their medications impact their health and well-being. “Our role as pharmacists in this department is non-traditional because we actually see patients in the exam rooms face-to-face,” said Cory Lankford, Ambulatory Care Clinical Pharmacist for Renown’s Anticoagulation Services. “We modify their medication regimens and drug recommendations under collaborative practice agreements.” “Because our role is so unique, we have a lot of opportunities to make a positive impact on patients,” added Janeen Abe, Ambulatory Care Clinical Pharmacist for Renown’s Anticoagulation Services. “We do a lot of direct patient interaction, including counseling patients on their medications and helping them navigate through their disease state.” “As a medical assistant in this department, we’re called the patient ‘liaisons’ to orchestrate who they should go to whether it’s a nurse, a provider or a pharmacist,” added Kiara Scruggs, Medical Assistant for Renown’s Anticoagulation Services. “We look at each patient’s medications and help with the Warfarin blood thinner monitor. We get to do a lot with patients." A key resource within the Pharmacy department and the emergency admission process, our Medical Reconciliation ("Med Rec") team stays on top of each patient's medication records. By ensuring each medication regimen is accurately reflected in each patient's chart and that patients continue to take their at-home medications while admitted to the hospital, this team provides vital insight into medications that could be a contributing factor to each patient's symptoms, including drug interactions. “Our medication reconciliation pharmacy technician team are true detectives,” said Heather Townsend, Clinical Pharmacy Supervisor. “When a patient arrives to the hospital, Med Rec works with patients, families, caregivers and outpatient pharmacies to compile a list of medications the patient has been taking a home. This list is used to make sure medications are not contributing to the patient’s symptoms and to assure medications are continued throughout the hospital stay. The addition of the medication reconciliation team has been one of the greatest advancements in medication safety.” “As a Med Rec Tech, we interview patients and family members and call pharmacies, skilled nursing facilities, etc. to obtain an accurate and complete medication list/history to outline what the patient is currently taking on a daily basis,” added Kara McGee, Medical Reconciliation Pharmacy Technician. “We make sure that we document the correct medication, dose, route, frequency and directions. This information is crucial because the nurses, pharmacists and physicians look at our work to figure out if any medications are contributing to the patient's health condition, and for the continuation of home medications on admission.” “Even though the Med Rec Tech might seem small in the hospital realm, it is very vital for patient information and beneficial to the patient's health,” added Brizza Villafan, Medical Reconciliation Pharmacy Technician. “There is never a dull moment in this work.” No matter the diagnosis, having Renown Pharmacy as an integral part of your healthcare team is a win-win situation for both you and them: you receive access to medication to help you heal, delivered to you with precision and care, and the pharmacy team has the opportunity to care for you and make a positive impact, a role they take seriously.
-
Exceptional Talent Joins William N. Pennington Cancer Institute at Renown; Dr. Kate Ward Brings Expertise and Vision as Vice President of Oncology Division
Renown Health is pleased to announce that Kate A. Ward, Pharm.D., BCPS, has been promoted to serve as Vice President, Oncology for Renown Regional Medical Center. Dr. Ward has over fourteen years of service to the organization, most recently as Director of Clinical Pharmacy responsible for the oversight of all clinical pharmacy activities at Renown Regional Medical Center, Renown South Meadows Medical Center, and Renown Rehabilitation Hospital. Additionally, Dr. Ward is the Residency Director of the Post-Graduate Year 1 (PGY1) Pharmacy Practice Residency at Renown. Over the last decade at Renown, Dr. Ward has served as Pharmacy Clinical Manager, Pharmacy Clinical Coordinator and Clinical Pharmacist. Dr. Ward will bring her pharmacy clinical service experience (including ICU, Outpatient Infusion, Pediatrics, Oncology and Emergency Services), Inpatient Care, Clinical Research, and Hospital Formulary Management, Electronic Medical Record Integration and Optimization to this important new role. As a dyad partner with Max J. Coppes, MD, PhD, MBA, Director of the William N. Pennington Cancer Institute at Renown, she will bring her clinical, operational, regulatory, and administrative experience to lead the oncology division. Dr. Ward, Dr. Coppes and the dedicated team will advance Renown’s mission to expand care, prevention, screening, research, and education with the goal of establishing the first National Institutes of Health, NCI designated Cancer Center for our State. “We are thrilled to welcome Dr. Ward as our new Vice President of Oncology. She brings a wealth of experience and a passion for advancing patient care. Dr. Ward will play a pivotal role in sharing the future our oncology division and furthering our commitment to providing exceptional care,” said Chris Nicholas, CEO of Renown Regional Medical Center. As a licensed pharmacist, Dr. Ward holds a Doctor of Pharmacy degree from the University of Colorado, Health Sciences Center. The Pharm.D. is a professional degree like a Doctor of Medicine (MD) or Doctor of Dental Surgery (DDS). As a doctorate, it represents the increasing responsibility pharmacists have in healthcare systems and the high trust Americans have in pharmacists. Dr. Ward completed her residency at Stanford Hospital and Clinics. She graduated from the University of Nevada, Reno with her bachelor’s degree in Nutritional Sciences. Dr. Ward is active in several professional associations and currently serves as a Pharmacy Board member for HealthTrust; Vice Chair of the American Society of Health System Pharmacists® Council on Therapeutics; and Vice Chair for the Silver State Scripts Board for the State of Nevada. Dr. Ward was the 2022 recipient of the Nevada Society of Health System Pharmacists (NVSHP) President’s Award. About Renown Health Renown Health is the region’s largest, locally governed, not-for-profit integrated healthcare network serving Nevada, Lake Tahoe and northeast California. With a diverse workforce of more than 7,000 employees, Renown has fostered a longstanding culture of excellence, determination and innovation. The organization comprises a trauma center, two acute care hospitals, a children’s hospital, a rehabilitation hospital, a medical group and urgent care network, and the locally owned not-for-profit insurance company, Hometown Health. To join the Renown Health team, visit renown.org/careers.
-
Study Shows Importance of Ensuring Participant and Provider Follow-up After a Genetic Screening Result
Released in partnership with the Desert Research Institute: New research from the Healthy Nevada Project® finds that a confirmed diagnosis does not always result in changes to patient care. Presenting individuals with potentially life-altering health information doesn’t mean the individuals – or their healthcare providers – will act on it. Follow-up education and conversations about actionable care plans with patients and their doctors are key next steps, according to new research from the Healthy Nevada Project. The Healthy Nevada Project is a genetic screening and research project that launched in 2016 as a partnership between DRI and Renown Health. The project now has more than 50,000 participants, with genetic sequencing provided by Helix. Between September 2018 and September 2020, the Healthy Nevada Project successfully notified 293 participants that they were genetically at risk for hereditary breast and ovarian cancer syndrome, Lynch syndrome, or familial hypercholesterolemia – three common genetic conditions known collectively as the Centers for Disease Control and Prevention (CDC) Tier 1 conditions. In a study published today in Frontiers in Genetics, Healthy Nevada Project scientists looked at the impact that notifying a patient of a positive finding for a CDC Tier 1 condition had on the care that the patient received in the months and years that followed. According to their results, among the 293 Healthy Nevada Project participants who were notified of their genetic risk of a CDC Tier 1 condition, 71 percent of participants with electronic health records shared their findings with healthcare providers. However, only 30 percent of the electronic health records for these patients contained documentation of the genetic diagnosis, and only 10 percent of examined patients experienced a possible change in care after receiving the results of their genetic screening. “The Healthy Nevada Project was implemented with a ‘hands-off’ approach where the participants receive their findings and decide with whom and when to share those findings. The findings were not automatically added to their electronic health records,” said Dr. Gai Elhanan, health data scientist at DRI and co-lead author of the study. “What we’re learning now is that to ensure that important genetic findings are integrated into the care journey it is important to make their inclusion into the electronic health records part of the study.” This study builds on previous Healthy Nevada Project research published in Nature Medicine demonstrating the importance of screening for CDC Tier 1 conditions, which affect about one in 75 individuals and can be mitigated or even prevented from developing into disease when detected early. This study found that as many as 90 percent of the CDC Tier 1 cases are missed by clinical providers during normal clinical care screenings and examinations. During the current study, the Healthy Nevada Project scientists found that 19 percent of studied participants had already developed one of the CDC Tier 1 conditions, and thus would have potentially benefited from earlier notification about their condition. The study team hopes that their findings will encourage individuals in Nevada to obtain genetic testing for these relatively common conditions. Even if individuals are older or have already suffered from diseases related to these conditions, testing could also prove beneficial to siblings, children, and grandchildren who may also be at risk and who could subsequently be screened in the event of a positive finding. The study team also encourages informing health care providers of the importance of incorporating genetic diagnoses into the pharmaceutical (for example, for Familial Hypercholesterolemia) and treatment advice given to patients. “As a result of this analysis, the clinicians at Renown Health and the Healthy Nevada Project researchers have made significant changes, including obtaining informed consent from participants to report positive findings from their genetics reports directly into their electronic medical record,” said Daniel Kiser, M.S., assistant research scientist of data science at DRI and co-lead author of the study. “This will help both participants, their clinical providers, and the whole state maximize the long-term benefits of the Healthy Nevada Project voluntary population-based genetic screening.” Additional information: The full text of the study, Incomplete Penetrance of Population-Based Genetic Screening Results in Electronic Health Record, is available from Frontiers in Genetics: https://www.frontiersin.org/articles/10.3389/fgene.2022.866169/full?&utm_source=Email_to_authors_&utm_medium=Email&utm_content=T1_11.5e1_author&utm_campaign=Email_publication&field=&journalName=Frontiers_in_Genetics&id=866169. This project was funded by Renown Health, the Renown Health Foundation, and the Nevada Governor’s Office of Economic Development. Study authors included Gai Elhanan (DRI), Daniel Kiser (DRI), Iva Neveux (DRI), Shaun Dabe (Renown Health), Alexander Bolze (Helix), William Metcalf (DRI), James Lu (Helix), and Joseph Grzymski (DRI/Renown Health). For more information on the Healthy Nevada Project® or to request genetic screening, please visit: https://healthynv.org/ About DRI The Desert Research Institute (DRI) is a recognized world leader in basic and applied environmental research. Committed to scientific excellence and integrity, DRI faculty, students who work alongside them, and staff have developed scientific knowledge and innovative technologies in research projects around the globe. Since 1959, DRI’s research has advanced scientific knowledge on topics ranging from humans’ impact on the environment to the environment’s impact on humans. DRI’s impactful science and inspiring solutions support Nevada’s diverse economy, provide science-based educational opportunities, and inform policymakers, business leaders, and community members. With campuses in Las Vegas and Reno, DRI serves as the non-profit research arm of the Nevada System of Higher Education. For more information, please visit www.dri.edu. About Renown Health Renown Health is the region’s largest, locally governed, not-for-profit integrated healthcare network serving Nevada, Lake Tahoe and northeast California. With a diverse workforce of more than 7,000 employees, Renown has fostered a longstanding culture of excellence, determination and innovation. The organization comprises a trauma center, two acute care hospitals, a children’s hospital, a rehabilitation hospital, a medical group and urgent care network, and the region’s largest, locally owned not-for-profit insurance company, Hometown Health. Renown is currently enrolling participants in the world’s largest community-based genetic population health study, the Healthy Nevada Project®. For more information, visit renown.org. About Helix Helix is the leading population genomics and viral surveillance company operating at the intersection of clinical care, research, and data analytics. Helix enables health systems, life sciences companies, payers, and government partners to accelerate the integration of genomic data into patient care and public health decision making. Learn more at www.helix.com.
-
Renown Institute Expands Partnership to Offer ELF Testing
Together, will test over 30,000 qualifying study participants by 2023 for risk of cirrhosis and liver-related illnesses. Renown Institute for Health Innovation (IHI) announced today that the organization and Gilead Sciences, Inc. will be joining forces with Siemens Healthineers to offer the Enhanced Liver Fibrosis (ELF™) Test to people with risks for nonalcoholic steatohepatitis (NASH) enrolled in the Healthy Nevada Project (HNP). The ELF Test will help identify people most at risk for progressing to cirrhosis and liver-related outcomes and allow healthcare providers to intervene before irreparable damage occurs. This noninvasive blood test uses three serum biomarkers to create an ELF score from a predefined algorithm, which can be used by doctors to help evaluate if a patient requires increased medical care and monitoring for their condition. Nonalcoholic fatty liver disease (NAFLD), which includes NASH, is prevalent in Nevada and under-diagnosed, likely affecting more than 500,000 adult Nevadans. If undetected and untreated, NASH can result in liver cirrhosis and may require liver transplantation or lead to death. There are more than 12,000 people on a waitlist for liver transplantation in the U.S. and this number continues to rise due to the increasing prevalence of NAFLD. “Thanks to important data collected through our Nonalcoholic Steatohepatitis Liver Disease Genome Atlas study, we now know that NASH is prevalent in the state of Nevada,” said Tony Slonim, M.D., DrPH, FACHE, president and CEO of Renown Health. “We are proud to expand our partnership with Gilead and begin working with Siemens Healthineers to improve health of those with liver disease and to take early detection one step further by offering Enhanced Liver Fibrosis, ELF testing for patients of Renown Health. This test provides our team of highly-skilled physicians an advanced, noninvasive method to actively assess dynamic liver fibrosis in study participants and intervene whenever necessary, contributing to a healthier Nevada.” “Gilead believes that noninvasive tests, including the ELF Test, will help improve the experience of people living with NASH. These tests may help to diagnose liver disease, monitor disease progression and evaluate responses to treatment without the requirement for liver biopsy,” said Rob Myers, MD, Vice President, Liver Fibrosis Clinical Research at Gilead Sciences. “The ELF Test has proven itself to be a valuable tool in NASH management and we hope this partnership will further support its use in routine care.” “We are very pleased that NASH patients in the Healthy Nevada Project now have access to the ELF Test which offers clinically useful prognostic information for their condition with the convenience of a simple blood test. Using our advanced laboratory expertise together with Renown IHI and Gilead, we can work towards better understanding of NASH and liver disease in a representative patient population,” said Sebastian Kronmueller, Head of Molecular Diagnostics at Siemens Healthineers. “We launched the Healthy Nevada Project to help people understand more about their health, to identify serious health risks, and to give people access to innovations like the ELF Test, so they can live their best lives,” said Renown’s chief scientific officer, Dr. Joseph Grzymski, who is also a research professor at the Desert Research Institute and principal investigator of the Healthy Nevada Project. It’s incredibly rewarding to be able to report clinical findings to help our 50,000 volunteer study participants, and to assist healthcare providers in helping their patients.” The provision of the ELF Test builds on a previously announced strategic collaboration between the Renown IHI and Gilead in July 2019. This ongoing partnership aims to collect and analyze de-identified genetic and electronic health data from 60,000 qualifying study participants to enhance the understanding of NAFLD and NASH and to potentially inform development of treatment options for these diseases. About NAFLD and NASH NAFLD is a build-up of fat in the liver of people who do not have a history of alcohol misuse. It is normal for the liver to contain some fat, but if more than 5 percent of the liver content is fat, it’s considered a fatty liver (steatosis). NASH is the most severe form of NAFLD in which a person has liver cell damage and inflammation of the liver. Inflammation and liver cell damage can cause fibrosis, or scarring of the liver, and can cause decreased liver function (1). The symptoms of NASH are often silent or non-specific, making it difficult to diagnose. About one-third of people with NASH develop cirrhosis or irreversible liver damage (2). About the ELF™ Test The ELF Test is a noninvasive blood test that can quickly identify which patients are at an elevated risk for developing cirrhosis and other liver-related clinical events (LREs). In contrast to standard liver enzyme tests that reflect liver damage that has already occurred, the ELF Test combines three serum direct biomarkers of active fibrosis. The ELF Test algorithm measures each of these biomarkers to create an ELF score, which can be used as an aid to assess the risk for future disease progression. Doctors may use this ELF score to help evaluate if a patient requires increased medical care and monitoring for their condition. Individuals interested in determining their risk for NASH and its progression are encouraged to enroll in the Nonalcoholic Steatohepatitis Liver Disease Genome Atlas study. Those who have already consented and participated in the study will be contacted with more information on how to receive an ELF blood test. For more information or to enroll, please contact RenownIHI@renown.org or (775) 982-6914. The Enhanced Liver Fibrosis (ELF™) Test kit is not available for sale in the U.S. Product availability may vary from country to country and is subject to varying regulatory requirements. In the U.S., the ELF Testing Service is available from Siemens Healthcare Laboratory, LLC (SHL), a CLIA-certified laboratory located in Berkeley, Calif. The ELF Testing Service, including the establishment of performance characteristics, was developed by SHL. The ELF Test has not been cleared or approved by the U.S. Food and Drug Administration. SHL is regulated under CLIA as qualified to perform high complexity testing. The ELF Test is used for clinical purposes and should not be regarded as investigational use only or research use only. About Renown Health Renown Health is the region’s largest, locally owned and governed, not-for-profit integrated healthcare network serving Nevada, Lake Tahoe and northeast California. With a diverse workforce of more than 7,000 employees, Renown has fostered a longstanding culture of excellence, determination and innovation. The organization comprises a trauma center, two acute care hospitals, a children’s hospital, a rehabilitation hospital, a medical group and urgent care network, and the region’s largest, locally owned not-for-profit insurance company, Hometown Health. Renown’s institute model addresses social determinants of health and includes: Child Health, Behavioral Health & Addiction, Healthy Aging and Health Innovation. Clinical institutes include: Cancer, Heart and Vascular Health, Neurosciences and Robotic Surgery. Renown is currently enrolling participants in the world’s largest community-based genetic population health study, the Healthy Nevada Project®. For more information visit, renown.org. About the Renown Institute for Health Innovation Renown Institute for Health Innovation is a collaboration between Renown Health - a locally governed and locally owned, not-for-profit integrated healthcare network serving Nevada, Lake Tahoe and northeast California; and the Desert Research Institute - a recognized world leader in investigating the effects of natural and human-induced environmental change and advancing technologies aimed at assessing a changing planet. Renown IHI research teams are focused on integrating personal healthcare and environmental data with socioeconomic determinants to help Nevada address some of its most complex environmental health problems; while simultaneously expanding the state’s access to leading-edge clinical trials and fostering new connections with biotechnology and pharmaceutical companies. Learn more at healthynv.org. Renown Health is Nevada’s most comprehensive and integrated healthcare network and maintains electronic health records for 1.02 million registered patients. In 2016, Renown Health and the Desert Research Institute established the Healthy Nevada Project (HNP), the nation’s first community-based population health study. In 2017 HNP began a partnership with Helix to leverage its population health services, Exome+™ sequencing, and consumer engagement tools. The HNP is now an ongoing collaboration between Renown IHI, the Desert Research Institute, a global leader in environmental data and applied research, and Helix, a personal genomics company. HNP combines genetic, environmental, social and clinical data to address individual and community health needs with the goal of improving health across the state and the nation. The HNP currently has over 60,000 participants. For more information, visit healthynv.org. About Siemens Healthineers Siemens Healthineers AG (listed in Frankfurt, Germany: SHL) is shaping the future of Healthcare. As a leading medical technology company headquartered in Erlangen, Germany, Siemens Healthineers enables healthcare providers worldwide through its regional companies to increase value by empowering them on their journey towards expanding precision medicine, transforming care delivery, improving the patient experience, and digitalizing healthcare. Siemens Healthineers is continuously developing its product and service portfolio, with AI-supported applications and digital offerings that play an increasingly important role in the next generation of medical technology. These new applications will enhance the company’s foundation in in-vitro diagnostic, image-guided therapy, and in-vivo diagnostics. Siemens Healthineers also provides a range of services and solutions to enhance healthcare providers ability to provide high-quality, efficient care to patients. In fiscal 2020, which ended on September 30, 2020, Siemens Healthineers, which has approximately 54,000 employees worldwide, generated revenue of €14.5 billion and adjusted EBIT of €2.2 billion. Further information is available at www.siemens-healthineers.com.Media Contact for Siemens Healthineers: Lance LongwellM: 610-448-6341E: lance.longwell@siemens-healthineers.com
Read More About Renown Institute Expands Partnership to Offer ELF Testing
-
Research Shows Genetic Approaches to Breast Cancer Screenings Yield More Accurate Results
Clinical researchers with the Healthy Nevada Project co-author research paper with findings that emphasize the need for a comprehensive approach to breast cancer risk assessment – including a focus on genetic medicine – to help ensure that individuals at high risk are identified and supported proactively rather than reactively. Breast cancer is a leading cause of cancer death among women in the United States. According to the American Cancer Society, about 1 in 8 women will develop breast cancer and about 1 in 39 women will die from breast cancer. Breast cancer is associated with increased age, hereditary factors, obesity, and alcohol use. Since 1990, breast cancer death rates have declined progressively due to advancements in treatment and detection. In Nevada there are an estimated 2,310 new breast cancer cases a year, and genetic mutations such as in the genes BRCA1 or BRCA2 remain a top risk factor for this prevalent disease. Recognizing the urgency for progress in breast cancer research, a collaborative effort between physicians, advanced practice providers and scientists from the Healthy Nevada Project® (HNP) and Helix have unveiled groundbreaking research. This study explores how genetic screenings are a necessary supplement to traditional testing methods, together offering more accurate insights into a patient's likelihood of developing breast cancer in the future. HNP is operated by Renown Genomic Medicine and the Institute for Health Innovation and is one of the largest community-based population health studies in the country. Their team works in collaboration with Helix, a leader in precision health that delivers comprehensive genomic solutions. Together, this dynamic partnership aims to understand breast cancer risk factors and pave the way for more effective preventative measures. The combined research team studied 25,591 female HNP participants to evaluate the performance of different genetic screening approaches to identify women at high risk of breast cancer. The results of this research suggest that a combined monogenic, or single-gene, and polygenic, or multi-gene, approach to breast cancer screenings helped produce more accurate results and more closely identify study participants who have a high genetic risk of developing the disease. "Based on this research, we are advocating a shift in approach which would improve breast cancer risk assessment through a combination of effective family history ascertainment and genetic screening,” said Joseph Grzymski, PhD, principal investigator of the Healthy Nevada Project, research professor at the University of Nevada, Reno School of Medicine and co-author of the breast cancer research paper. “This tailored approach, founded on the assessment of individual genetic risk, not only intends to elevate patient well-being but also will improve efficiency and equity in healthcare." Complementing the team’s research on leveraging genetics to identify women at low genetic risk of breast cancer that could safely defer mammogram screenings by five to 10 years that was released in late 2023 in JAMA Oncology, the study suggests that incorporating genetic information can assist in personalizing breast cancer screenings and optimizing the use of screening resources. "Existing disparities persist across various facets of breast cancer screening and treatment; however, genetic screening is clearly a powerful tool to help facilitate early intervention for those at higher risk,” said Jamie Schnell Blitstein, APRN, a primary care nurse practitioner at Renown Health and co-author of the breast cancer research paper. “By placing a heightened focus on risk, we underscore the pivotal role of preventative breast cancer screening.” Despite the availability of effective methods for early screening, co-authors of this research found that 78 percent of women with a family history of breast cancer had their risk ascertained only after a breast cancer diagnosis. The findings emphasize the need for a comprehensive approach to breast cancer risk assessment – including a focus on genetic medicine – to help ensure that individuals at high risk are identified and supported proactively rather than reactively. “These findings that can profoundly impact how healthcare is delivered were only made possible by all the participants who were willing to consent to research,” said Alex Bolze, PhD from Helix and co-author of the publication. “Broad-scale collaboration projects like these between Renown Health and UNR that engage large populations where participants share both their genetic information as well as electronic health records drive advancements in preventative medicine, as well as fundamental biological research.” The research paper was officially accepted on Jan. 29, 2024, and will be published by Elsevier, Inc. on behalf of the American College of Medical Genetics and Genomics. The contents of the paper will appear in the international journal Genetics in Medicine Open. Read the full article by visiting sciencedirect.com. The Healthy Nevada Project is currently recruiting new study participants. Free to all Nevadans with a saliva sample or blood draw, participants and their referring providers receive access to whole-exome sequencing and clinical grade results that help provide insight into their unique genetic risks tied to heart disease and certain cancers. If you are interested in enrolling in the study, schedule a Virtual Consent Appointment through MyChart or contact the Renown Institute for Health Innovation at RenownIHI@renown.org or (775) 982-6914 to be connected to a Genomic Representative. About Renown Health Renown Health is the region’s largest, not-for-profit integrated healthcare network serving Nevada, Lake Tahoe and northeast California. With a diverse workforce of more than 7,000 employees, Renown has fostered a longstanding culture of excellence, determination and innovation. The organization comprises a trauma center, two acute care hospitals, a children’s hospital, a rehabilitation hospital, a medical group and urgent care network, and the region’s largest, locally owned not-for-profit insurance company, Hometown Health. Renown is currently enrolling participants in the largest community-based genetic population health study, the Healthy Nevada Project®. To join the Renown Health team, visit renown.org/careers. About Helix Helix is the leading population genomics and viral surveillance company operating at the intersection of clinical care, research, and data analytics. Helix enables health systems, life sciences companies, payers, and government partners to accelerate the integration of genomic data into patient care and public health decision-making. Learn more at helix.com.
-
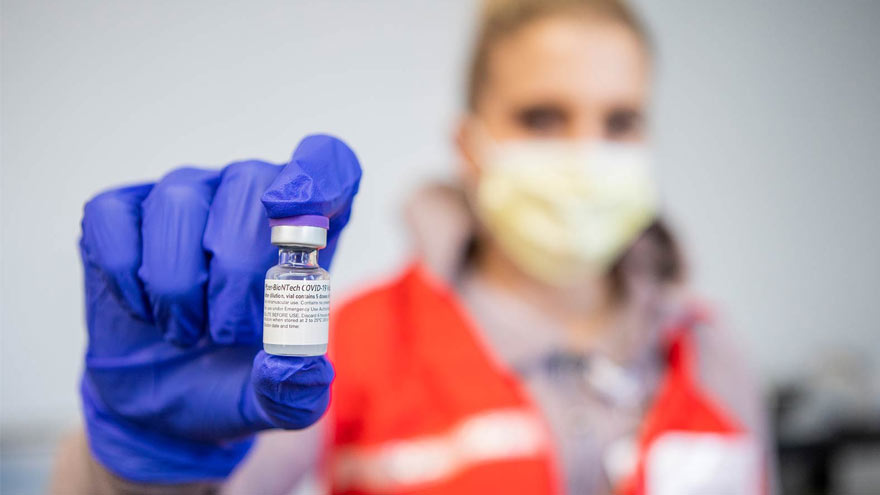
Pharmacists Answer Questions about the COVID-19 Vaccines
Vaccines that provide protection against the COVID-19 virus are bringing us closer to the end of this deadly pandemic. Two different COVID-19 vaccines are currently available in the U.S. today: one from Pfizer and the other from Moderna. Kate Ward, PharmD, BCPS, Director of Clinical Pharmacy at Renown Health and Adam Porath, PharmD, Vice President of Pharmacy at Renown, share what you need to know about these vaccines. When two COVID-19 vaccines were approved by the U.S. Food & Drug Administration (FDA) in December 2020, it was cause for celebration. Why? Because according to the CDC, the vaccines are 94 percent or more effective in providing protection against the COVID-19 virus! Many people are seeking information about the new Moderna and Pfizer vaccines. Below, our pharmacy leaders provide answers to some commonly asked questions. How do the COVID-19 Vaccines Work? The Pfizer and Moderna vaccines are both mRNA vaccines that help your immune system develop antibodies against the COVID-19 virus. The vaccines use messenger RNA, or mRNA, to show our bodies’ protein-making cells how to make the spike proteins of the COVID-19 virus. Our immune system reacts to these spike proteins by creating antibodies that can recognize and destroy them. So when a person is exposed to the virus in the future, they will be less likely to get sick. What are the Differences between the Pfizer and Moderna Vaccines? The Pfizer and Moderna COVID-19 vaccines are very similar, with just a few small differences worth noting. The main difference between the two vaccines is when you should receive your follow-up dose. Patients who receive a first dose of Pfizer should receive their second dose about three weeks later. Those who receive a first dose of Moderna should receive their follow-up vaccination roughly four weeks after their first dose. People 18 years and older can receive the Moderna vaccine while people 16 years and older can receive the Pfizer vaccine. Dosage for the Moderna vaccine is 0.5 ml (100 mcg). Dosage for the Pfizer vaccine is 0.3 ml (30 mcg).
Read More About Pharmacists Answer Questions about the COVID-19 Vaccines