Search
-
Research Shows Genetic Approaches to Breast Cancer Screenings Yield More Accurate Results
Clinical researchers with the Healthy Nevada Project co-author research paper with findings that emphasize the need for a comprehensive approach to breast cancer risk assessment – including a focus on genetic medicine – to help ensure that individuals at high risk are identified and supported proactively rather than reactively. Breast cancer is a leading cause of cancer death among women in the United States. According to the American Cancer Society, about 1 in 8 women will develop breast cancer and about 1 in 39 women will die from breast cancer. Breast cancer is associated with increased age, hereditary factors, obesity, and alcohol use. Since 1990, breast cancer death rates have declined progressively due to advancements in treatment and detection. In Nevada there are an estimated 2,310 new breast cancer cases a year, and genetic mutations such as in the genes BRCA1 or BRCA2 remain a top risk factor for this prevalent disease. Recognizing the urgency for progress in breast cancer research, a collaborative effort between physicians, advanced practice providers and scientists from the Healthy Nevada Project® (HNP) and Helix have unveiled groundbreaking research. This study explores how genetic screenings are a necessary supplement to traditional testing methods, together offering more accurate insights into a patient's likelihood of developing breast cancer in the future. HNP is operated by Renown Genomic Medicine and the Institute for Health Innovation and is one of the largest community-based population health studies in the country. Their team works in collaboration with Helix, a leader in precision health that delivers comprehensive genomic solutions. Together, this dynamic partnership aims to understand breast cancer risk factors and pave the way for more effective preventative measures. The combined research team studied 25,591 female HNP participants to evaluate the performance of different genetic screening approaches to identify women at high risk of breast cancer. The results of this research suggest that a combined monogenic, or single-gene, and polygenic, or multi-gene, approach to breast cancer screenings helped produce more accurate results and more closely identify study participants who have a high genetic risk of developing the disease. "Based on this research, we are advocating a shift in approach which would improve breast cancer risk assessment through a combination of effective family history ascertainment and genetic screening,” said Joseph Grzymski, PhD, principal investigator of the Healthy Nevada Project, research professor at the University of Nevada, Reno School of Medicine and co-author of the breast cancer research paper. “This tailored approach, founded on the assessment of individual genetic risk, not only intends to elevate patient well-being but also will improve efficiency and equity in healthcare." Complementing the team’s research on leveraging genetics to identify women at low genetic risk of breast cancer that could safely defer mammogram screenings by five to 10 years that was released in late 2023 in JAMA Oncology, the study suggests that incorporating genetic information can assist in personalizing breast cancer screenings and optimizing the use of screening resources. "Existing disparities persist across various facets of breast cancer screening and treatment; however, genetic screening is clearly a powerful tool to help facilitate early intervention for those at higher risk,” said Jamie Schnell Blitstein, APRN, a primary care nurse practitioner at Renown Health and co-author of the breast cancer research paper. “By placing a heightened focus on risk, we underscore the pivotal role of preventative breast cancer screening.” Despite the availability of effective methods for early screening, co-authors of this research found that 78 percent of women with a family history of breast cancer had their risk ascertained only after a breast cancer diagnosis. The findings emphasize the need for a comprehensive approach to breast cancer risk assessment – including a focus on genetic medicine – to help ensure that individuals at high risk are identified and supported proactively rather than reactively. “These findings that can profoundly impact how healthcare is delivered were only made possible by all the participants who were willing to consent to research,” said Alex Bolze, PhD from Helix and co-author of the publication. “Broad-scale collaboration projects like these between Renown Health and UNR that engage large populations where participants share both their genetic information as well as electronic health records drive advancements in preventative medicine, as well as fundamental biological research.” The research paper was officially accepted on Jan. 29, 2024, and will be published by Elsevier, Inc. on behalf of the American College of Medical Genetics and Genomics. The contents of the paper will appear in the international journal Genetics in Medicine Open. Read the full article by visiting sciencedirect.com. The Healthy Nevada Project is currently recruiting new study participants. Free to all Nevadans with a saliva sample or blood draw, participants and their referring providers receive access to whole-exome sequencing and clinical grade results that help provide insight into their unique genetic risks tied to heart disease and certain cancers. If you are interested in enrolling in the study, schedule a Virtual Consent Appointment through MyChart or contact the Renown Institute for Health Innovation at RenownIHI@renown.org or (775) 982-6914 to be connected to a Genomic Representative. About Renown Health Renown Health is the region’s largest, not-for-profit integrated healthcare network serving Nevada, Lake Tahoe and northeast California. With a diverse workforce of more than 7,000 employees, Renown has fostered a longstanding culture of excellence, determination and innovation. The organization comprises a trauma center, two acute care hospitals, a children’s hospital, a rehabilitation hospital, a medical group and urgent care network, and the region’s largest, locally owned not-for-profit insurance company, Hometown Health. Renown is currently enrolling participants in the largest community-based genetic population health study, the Healthy Nevada Project®. To join the Renown Health team, visit renown.org/careers. About Helix Helix is the leading population genomics and viral surveillance company operating at the intersection of clinical care, research, and data analytics. Helix enables health systems, life sciences companies, payers, and government partners to accelerate the integration of genomic data into patient care and public health decision-making. Learn more at helix.com.
-
Gilead Sciences and Renown Institute for Health Innovation Announce Strategic Collaboration to Advance Understanding of Nonalcoholic Steatohepatitis (NASH)
Gilead Sciences, Inc. (Nasdaq: GILD) and the Renown Institute for Health Innovation (IHI) today announced a strategic collaboration to collect and analyze genetic and electronic health data that can enhance the understanding of nonalcoholic steatohepatitis (NASH) and potentially inform development of treatment options for the disease. Under the terms of the collaboration and license agreement, Gilead will provide funding to Renown IHI to sequence and analyze the DNA of 15,000 individuals living with NASH or nonalcoholic fatty liver disease (NAFLD) as well as a control cohort of 40,000 individuals in Nevada. “Combining the sequencing of protein coding DNA, with extensive electronic health record data will enable a deep analysis of the roles of genetics and environment in NASH incidence and progression,” said John McHutchison, AO, MD, Chief Scientific Officer and Head of Research and Development, Gilead Sciences. “The analysis of these large datasets in collaboration with Renown IHI could help identify genetic variants that impact the risk of developing NASH and thereby advance the discovery and development of new treatments for this disease.” Renown Health is Nevada’s most comprehensive and integrated healthcare network and maintains electronic health records for 1.02 million registered patients. In 2016, Renown Health and the Desert Research Institute established the Healthy Nevada Project (HNP), the nation’s first community-based population health study. In 2017 HNP began a partnership with Helix to leverage its population health services, Exome+™ sequencing, and consumer engagement tools. The HNP is now an ongoing collaboration between Renown IHI, the Desert Research Institute, a global leader in environmental data and applied research, and Helix, a personal genomics company. HNP combines genetic, environmental, social and clinical data to address individual and community health needs with the goal of improving health across the state and the nation. The HNP currently has 40,000 participants. “Combining genetic sequencing with large sets of data can play a critical role in understanding and identifying serious health risks, including diseases like NASH. We are excited to collaborate with Gilead to better understand the condition and its complexities,” said Anthony Slonim, MD., DrPH. “Any genetic variants identified in participants through the collaboration may be shared with the participants for patient care purposes.” About NASH Nonalcoholic steatohepatitis (NASH) is a chronic form of liver disease characterized by excess fat in the liver, inflammation, and liver cell damage. Inflammation and liver cell damage can cause scarring of the liver, or fibrosis, and ultimately lead to cirrhosis or liver cancer. NASH is more common in people with certain conditions, including obesity and type 2 diabetes. There are currently limited approved treatments for patients living with NASH. About Gilead Sciences Gilead Sciences, Inc. is a research-based biopharmaceutical company that discovers, develops and commercializes innovative medicines in areas of unmet medical need. The company strives to transform and simplify care for people with life-threatening illnesses around the world. Gilead has operations in more than 35 countries worldwide, with headquarters in Foster City, California. For more information on Gilead Sciences, please visit the company’s website at www.gilead.com. About Renown Health Renown Health is a locally governed and locally owned, not-for-profit integrated healthcare network serving Nevada, Lake Tahoe and northeast California. Renown is one of the region’s largest private employers with a workforce of more than 7,000. It comprises three acute care hospitals, a rehabilitation hospital, the area’s most comprehensive medical group and urgent care network, and the region’s largest and only locally owned not-for-profit insurance company, Hometown Health. Renown Health’s commitment has extended beyond traditional health care to include community health and well-being. Renown Health works to improve health care through science, research and genetics; forge community partnerships that improve lives and develop innovative models that are improving health care in Nevada. For more information, visit renown.org. About Helix Helix’s mission is to empower every person to improve their life through DNA. Helix is accelerating the integration of genomic data into clinical care and broadening the impact of large-scale population health programs by providing comprehensive expertise in DNA sequencing, bioinformatics, and individual engagement. Powered by their proprietary Exome+™ assay—a panel-grade exome enhanced by more than 300,000 informative non-coding regions—Helix offers health systems a scalable solution which enables the discovery of medically relevant, potentially life-saving, genetic information. Additionally, Helix offers a suite of DNA-powered products for continued individual engagement and discovery. Helix is headquartered in the San Francisco Bay Area and has one of the world’s largest CLIA-certified, CAP-accredited Next Generation Sequencing labs, located in San Diego, California. Learn more at www.helix.com. Helix, the Helix logo, and Exome+ are trademarks of Helix Opco, LLC. Gilead Forward-Looking Statement This press release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that are subject to risks, uncertainties and other factors, including the risk that the parties may not realize the potential benefits of this collaboration, and Gilead may fail to discover, develop and commercialize any product candidates for the treatment of NASH. All statements other than statements of historical fact are statements that could be deemed forward-looking statements. These risks, uncertainties and other factors could cause actual results to differ materially from those referred to in the forward-looking statements. The reader is cautioned not to rely on these forward-looking statements. These and other risks are described in detail in Gilead’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2019, as filed with the U.S. Securities and Exchange Commission. All forward-looking statements are based on information currently available to Gilead, and Gilead assumes no obligation to update any such forward-looking statements. For more information on Gilead Sciences, please visit the company’s website at www.gilead.com, follow Gilead on Twitter (@GileadSciences) or call Gilead Public Affairs at 1-800-GILEAD-5 or 1-650-574-3000. Additional Media Contact: Sung Lee, Investors 650-524-7792 Arran Attridge, Media 650-425-8975
-
Researchers at Renown Seek Convalescent Plasma Study Participants
Physician researchers seek to understand how the immune system responds to COVID-19 and create a healthier Nevada. During the early stages of the pandemic, convalescent plasma was considered the only viable treatment option available for patients with COVID-19. Convalescent plasma is the component of the blood from recovered patients that may contain COVID-19 antibodies that help fight the infection. The National Institutes of Health has since developed treatment guidelines for COVID-19 based on clinical trial data and many studies are still underway worldwide assessing various additional treatment options. Convalescent plasma was in high demand but difficult to locate for COVID-19 patients in the northern Nevada area. A 24-year-old nursing assistant, Austin Meegan, was hospitalized and spent weeks staving off kidney and lung failure before learning he was eligible for an experimental blood transfusion that showed promise in treating COVID-19. Doctors estimated Meegan had only about a 3% chance of tracking down a donor to match his rare blood type. A COVID-19 survivor, Thomas Gibson, a Texas resident with the same blood type, traveled to Reno to donate his viral antibodies and a convalescent plasma donation credited with helping to save Meegan’s life. Physician clinical researchers and scientists at Renown and the University of Nevada, Reno School of Medicine (UNR Med) knew they needed to create better options for patients and physicians. Clinical researchers developed a study to help other patients like Austin, and pleaded for donations from recovered COVID-19 patients to donate their convalescent plasma. The researcher teams looked to understand how the body’s immune system responds to the virus over time, to aid them in developing new treatments for COVID-19. “The world’s capacity to get through the COVID crisis will depend on four things — science, technology, innovation and partnerships, says Tony Slonim, MD, DrPH, President and CEO of Renown Health. “Taking lab bench discoveries to the bedside of patients in an efficacious and timely manner is not easy, but with UNR Med and our partners, we are making great strides in advancing clinical research which has the power to save lives and to create a healthier Nevada.” “It’s tremendously promising to partner on clinical research that will not only help us better understand the disease, but help inform treatment for those combatting COVID-19 that has had such a devastating impact on Nevadans, our nation and the world,” says UNR Med Dean Thomas L. Schwenk, MD. Renown, UNR Med and other area health care partners collaborated with Vitalant to collect plasma from recovered donors for a study on the treatment's efficacy. Eligible donors are at least 18 years old, weigh more than 110 pounds and are healthy. Donors had fully recovered from a confirmed diagnosis of COVID-19. Project coordinators at the Renown Research Office were overwhelmed by the community’s support and plasma blood donations. Additional partnerships with the Washoe County Health District, the State of Nevada and the Governor’s office, Saint Mary’s Medical Center, Northern Nevada Medical Center, Carson Tahoe Health and the VA Sierra Nevada Health Care System, along with many area health care providers helped the team meet their goals of enrolling 120 eligible participants in the study. “Our success in this study rests heavily on the support of our great community, as well as the innovation and collaboration demonstrated by Renown and UNR Med,” said Sara Healy, MD, MPH, principal investigator of the study and a pediatric infectious disease physician at Renown Children’s Hospital and UNR Med. “We are proud to be at the forefront of conducting essential research during such a pivotal time in history, and look forward to our continued partnership as we continue this important work.” “The control of COVID-19 in our communities relies on testing. The study that is being launched to develop a sensitive, specific and easier way to collect specimens (blood) is advancing the field and brings promise towards getting to our common goal of having the right diagnostic test for the right clinical situation at the right time,” says Mark Riddle, MD, DrPH, FISTM, associate investigator of the study and associate dean for clinical research and professor at UNR Med's Department of Internal Medicine and Medical Research. The research team is now asking area residents to participate in a study to analyze the efficacy of two COVID-19 tests. Participants will undergo two blood tests: one being a finger stick to provide results for a rapid test, and the other is a traditional venipuncture draw confirming the presence or absence of COVID-19 antibodies. This study is a collaboration with InBios International, Inc., a leading biotechnology company based in Seattle. Researchers are seeking: Individuals who have confirmed positive for COVID-19 and who have recently recovered from the virus. Study participants must be within 7-28 days from the onset of their symptoms. Individuals who have recently tested negative for COVID-19 and have never tested positive. Those who are interested in participating in the study may contact project coordinators at the Renown Research Office at (775) 982-3646, or via e-mail at covidplasmascreening@renown.org, 7:30 A.M. – 5:00 P.M., Monday through Friday. Individuals aged 18-75 in general good health are encouraged to consider participating in this ongoing study. There is no cost to participate in this study and participation is voluntary. An individual’s decision to participate will not affect their current or future relations with their health care provider(s), health district, or the community. Those who decide to participate are free to withdraw at any time. “Time is of the essence with COVID. If we can get test results to people and their clinicians in a more timely way, we can make a faster diagnosis of a patient's condition, says Christopher M. Kozlowski, MD, MHA, Renown's institutional research officer and Medical Director/VP of Renown Institute for Heart & Vascular Health. “As we refine the accuracy of our testing; we are applying sensitivity and specificity testing for true negative and true positive results. This provides people with more timely and accurate results and better quality care.” About Renown Health Renown Health is a locally governed, not-for-profit integrated healthcare network serving northern Nevada, Lake Tahoe and northeast California. Renown is one of the region’s largest private employers with a workforce of more than 7,000. It comprises three acute care hospitals, a rehabilitation hospital, the area’s most comprehensive medical group and urgent care network, and the region’s largest and only locally owned not-for-profit insurance company, Hometown Health. Renown has a long tradition and commitment to improve the care and the health of our community. About the University of Nevada, Reno School of Medicine The University of Nevada, Reno School of Medicine (UNR Med), Nevada’s first medical school, is a community-based, research-intensive medical school with a statewide vision for a healthy Nevada. Established in 1969, UNR Med is improving the health and well-being of all Nevadans and their communities through excellence in student education, postgraduate training and clinical care, research with local, national and global impact and a culture of diversity and inclusion. For more information, visit med.unr.edu.
Read More About Researchers at Renown Seek Convalescent Plasma Study Participants
-
Introducing Dana Renown Institute for Health Innovations New Life Sized Holographic Kiosk
Presented by the Desert Research Institute and Renown Health, DANA will help people learn more about the Healthy Nevada Project® and their own unique, genetic health traits What is the Healthy Nevada Project®? What are the benefits of joining this research study? How can I find out if I carry genes for health risks like heart disease, hereditary breast and ovarian cancer syndrome and Lynch syndrome? What if a holographic avatar, powered by artificial intelligence (AI), could answer all these questions and more? DANA has all the answers, she is a virtual assistant with “DNA” in her name, presented by the Renown Institute for Health Innovation (Renown IHI), a collaboration between Renown Health and the Desert Research Institute (DRI). This life-sized, holographic avatar will greet individuals outside Renown Regional Medical Center’s Sierra Café, and talk to them about the Healthy Nevada Project, the world’s largest community-based genetic population health study. Members of the media are invited to meet DANA this morning from 10 a.m. – noon. Please reply to this email or call (775) 691-7308 to coordinate a meet and greet. “Unfortunately, Nevada ranks among the lowest in the nation for health outcomes—and we are working to change that,” said Anthony Slonim, M.D., D.Ph., president and CEO of Renown Health and president of Renown Institute for Health Innovation. “Through the Healthy Nevada Project® , our goal is to offer genetic testing to every Nevadan interested in learning more about their health and genetic profile. Thanks to this advanced technology, DANA will offer people a personalized explanation of the Project, and help them take the next step to better understand their health, and their health risks, so they can modify their behavior and ultimately, live a healthier, happier life.” With more than 51,000 study participants enrolled to date, the Healthy Nevada Project® is considered the fastest-enrolling genetic study in the country. The Project is also the first of its kind to return clinical results to study volunteers, which means participants can learn their genetic risks tied to heart disease and certain cancers, as well as lifestyle changes that could potentially help reduce their risk and prevent disease. Furthermore, participants can choose to share their information with their medical provider to improve and enhance their medical care. “We are always happy to engage with our study participants and look forward to having them meet and engage with DANA,” said Joseph Grzymski, Ph.D., research professor at DRI, principal investigator of the Healthy Nevada Project® and chief scientific officer for Renown Health. “At a time of physical distancing and limiting human contact where possible, using tools like an avatar and AI are important for communicating, whether it be for genetics, vaccinations or other important health information.” Visitors can interact with DANA through a touch screen (cleaned and sanitized after every encounter) to learn more about the study, enter their contact information and schedule an appointment to join the free genetics study or receive more information about their test results. Kiosk visitors are asked to maintain physical distance guidelines and use the hand sanitizer and Sani Wipes available next to the kiosk. Renown Institute for Health Innovation is a collaboration between Renown Health - a locally governed and locally owned, not-for-profit integrated healthcare network serving Nevada, Lake Tahoe and northeast California; and the Desert Research Institute - a recognized world leader in investigating the effects of natural and human-induced environmental change and advancing technologies aimed at assessing a changing planet. Renown IHI research teams are focused on integrating personal healthcare and environmental data with socioeconomic determinants to help Nevada address some of its most complex environmental health problems; while simultaneously expanding the state’s access to leading-edge clinical trials and fostering new connections with biotechnology and pharmaceutical companies. Learn more at healthynv.org.
-
Population Genetic Screening Show to Efficiently Identify Increased Risk for Inherited Disease
Healthy Nevada Project’s community-based approach reveals up to 90% of CDC Tier 1 genetic condition risks missed using clinical care guidelines. In a new study published today in the journal Nature Medicine, researchers behind the Healthy Nevada Project® suggest that community-based genetic screening has the potential to efficiently identify individuals who may be at increased risk for three common inherited genetic conditions known to cause several forms of cancer and increased risk for heart disease or stroke. In 2018, the Healthy Nevada Project® (the largest, community-based population health study combining genetic, clinical, environmental and social data) started notifying consenting study participants who have certain genetic variants which predispose them to the Centers for Disease Control and Prevention (CDC) Tier 1 genetic conditions. The study focused on identifying carriers of these conditions, which include Hereditary Breast and Ovarian Cancer, Lynch Syndrome, and Familial Hypercholesterolemia, because they are the most common conditions and early detection and treatment could significantly lower morbidity and mortality. Initial results from almost 27,000 study participants showed that 90% of carriers of the CDC Tier 1 genetic conditions were not previously identified in a clinical setting. The authors conclude that population genetic screening would identify at-risk carriers not identified during routine care. “Our first goal was to deliver actionable health data back to the participants of the study and understand whether or not broad population screening of CDC Tier 1 genomic conditions was a practical tool to identify at-risk individuals,” explained Joseph Grzymski, Ph.D., the principal investigator of the Healthy Nevada Project®, a research professor at the Desert Research Institute (DRI), chief scientific officer for Renown Health and lead author of the study. “Now, two years into doing that, it is clear that the clinical guidelines for detecting risk in individuals are too narrow and miss too many at risk individuals.” Within the group of 26,906 Healthy Nevada Project® participants that Grzymski’s research team studied, 358 (1.33%) were carriers for CDC Tier 1 conditions. However, only 25% of those individuals met clinical guidelines for genetic screening. Additionally, more than 20% of the carriers already had a diagnosis of disease relevant to their underlying genetic condition. “We’re at a point now where it’s possible to do clinical-grade genetic screening at population-scale,” added James Lu, M.D. Ph.D., co-founder and chief scientific officer of Helix and senior co-author of the study. “What this study demonstrates is the potential impact of doing so. By making genetic screening available more broadly, we can help the millions of Americans who are unaware that they are living at increased risk for highly actionable, genetic conditions take action.” Most notably, the study found that of the 273 participants who were carriers of the CDC Tier 1 genetic conditions and had clinical record information, only 22 individuals showed any previous suspicion of their underlying genetic conditions. “For the first time, we are providing information at the individual level so study participants can make lifesaving changes to reduce their risk based on their genetics,” said Anthony Slonim, M.D., Dr.PH., FACHE, president and CEO of Renown Health and co-director of the Project® study. “We’re conducting research on the community level to develop leading-edge research on health determinants for entire neighborhoods, states and eventually, the country. Returning these results allows us to understand the prevalence of genetically programmed diseases and illnesses that we have here in Nevada and ensure we are providing the best prevention and care plans. For the individual, the return of results can be life changing.” According to the CDC, early detection and intervention of the Tier 1 genetic conditions could have a meaningful potential for clinical action ability and a positive impact on public health. The Healthy Nevada Project®, which launched in 2016, offers free genetic testing to every Nevadan, aged 18 and older, interested in learning more about their health and genetic profile. With more than 50,000 study participants enrolled in four years, the Healthy Nevada Project® has become the fastest-enrolling genetic study in the world. For more about the Healthy Nevada Project® please visit healthynv.org Renown Institute for Health Innovation is a collaboration between Renown Health – a locally governed and locally owned, not-for-profit integrated healthcare network serving Nevada, Lake Tahoe and northeast California; and the Desert Research Institute – a recognized world leader in investigating the effects of natural and human induced environmental change and advancing technologies aimed at assessing a changing planet. Renown IHI research teams are focused on integrating personal healthcare and environmental data with socioeconomic determinants to help Nevada address some of its most complex environmental health problems; while simultaneously expanding the state’s access to leading-edge clinical trials and fostering new connections with biotechnology and pharmaceutical companies. Learn more at Healthynv.org. Helix is the leading population genomics company operating at the intersection of clinical care, research, and genomics. Its end-to-end platform enables health systems, life sciences companies, and payers to advance genomic research and accelerate the integration of genomic data into clinical care. Powered by one of the world's largest CLIA / CAP next-generation sequencing labs and its proprietary Exome+Ⓡ assay, Helix supports all aspects of population genomics including recruitment and engagement, clinically actionable disease screening, return of results, and basic and translational research. In response to the COVID-19 public health crisis, Helix has launched a sensitive and scalable end-to-end COVID-19 test system to meet the needs of health systems, employers, governments, and other organizations across the country. Learn more at www.helix.com.
-
COVID-19 Booster Shots, What You Need to Know
Getting the COVID-19 booster is the best way to protect yourself from severe illness or death due to COVID-19, and both the CDC and the FDA have approved booster shots for people ages 18 and older. So, with the holidays right around the corner and infection rates on the rise both in Nevada and nationally, the best thing you can do to prevent the continued spread of this deadly virus is to get boosted today. The Basics: Who: It is recommended that everyone 18 years or older get a COVID-19 booster shot. When: At least 6 months after completing your primary COVID-19 vaccination series. What: Any of the COVID-19 vaccines authorized in the United States. The CDC allows for mix and match dosing for booster shots. How: To make an appointment for your COVID-19 vaccine booster, please visit vaccines.gov today. Appointment Reminders: Don’t forget to bring your CDC vaccination record card to your appointment. Refresh yourself on the potential side effects and remember that these are normal signs your body is building up protection. Commonly Asked Questions: Q: Does anything change if I received the Johnson & Johnson as my first COVID-19 vaccine? A: If you received the Johnson & Johnson COVID-19 vaccine, you are elidable for a booster two months after completing your primary vaccine. Q: Is the formula the same for the boosters as it was for the primary vaccine? A: COVID-19 booster shots are the same formulation as the current COVID-19 vaccines. However, in the case of the Moderna COVID-19 vaccine booster shot, it is half the dose of the vaccine people get for their primary series. Q: Am I still considered “fully vaccinated” if I don’t receive a COVID-19 booster shot. A: Yes, everyone is still considered fully vaccinated two weeks after their second dose in a two-shot series, such as the Pfizer-BioNTech or Moderna vaccines, or two weeks after a single-dose vaccine, such as the J&J/Janssen vaccine. All information courtesy of the Center for Disease Control and Prevention. All information courtesy of the Center for Disease Control and Prevention
Read More About COVID-19 Booster Shots, What You Need to Know
-
Reno Widow Inspires New Visitor Policy for Renown
Renown Health is one of the country’s first health systems to lift visitor restrictions for patients with COVID-19 and encourage the family to be at the patient’s bedside. Read Darlene and Dave’s story to understand why we’re updating our visitor policy. Dave and Darlene Randolph found joy in exploring antique shops and garage sales to find damaged or discarded vintage pieces. Dave would spend many hours scraping, cleaning, sanding, and refinishing items, transforming them into functional, beautiful pieces of furniture. Every piece in their home rekindles a memory and has a story to tell. On Thanksgiving, when Dave was too ill to gather around their antique dining room table, Darlene called the ambulance. Ailing with COVID-19 for two weeks, Dave had not been improving. When the EMTs reached her home and asked Darlene what underlying conditions he had, she said, “all of them.” David was seriously ill. Hospitalized for COVID-19, their communications options were limited. The only way Darlene could communicate with Dave was on a video call or by telephone. Dave spent 17 days hospitalized at Renown Regional Medical Center in Reno. Darlene spent 17 days waiting by the phone for more information on his condition. Darlene said he had “up days and down days,” but thought he might be home, sitting at their antique dinner table for Christmas. Sadly, Dr. David Randolph lost his battle with COVID-19 on December 13, 2020, and died as he slept in a hospital bed. When Darlene wrote his obituary for the newspaper, she gave thanks to the “tremendous nurses and doctors at Renown Regional Medical Center, for providing his care during a time when the family could not be with him.” Taking Action to Inspire Change Darlene wished she could have been there. Over their 45-year marriage, she had always been there. Darlene said, “I had always been at his bedside, as his advocate, to help communicate and straighten things out.” As a registered dietician, she worked in hospitals, knew the protocol, and knew that Renown had a restricted visitor policy to stop the virus’s spread- to other patients, staff, and their family members. Still, she wished she could have spent more time with him. On Christmas Eve, she sat down and wrote to Renown leadership. “As the wife of a COVID patient who recently passed away in your hospital, I want to express my thanks to you and your staff for the care he received in the last days of his life. I am aware that the nurses and staff are working under dangerous conditions and risking their health and lives by caring for multiple COVID patients. The staff is gracious, concerned, and doing everything they can.” She continued, “I know procedures are changing every hour to try to stay ahead of this dangerous virus, and I am sharing my experiences, hoping they will be helpful when establishing policies that impact families.” Darlene explained that despite receiving assurances that Dave’s nurse or a doctor would call daily, sometimes they would forget. She explains in her letter, “how important it is, in these times when the family cannot visit, and has only infrequent communication and is anxiously waiting at home for information about their loved one, how much it means to get a call from someone caring for him at the hospital. If there is a way you can help assure nurses have time to make calls or assist patients in making calls because it is an important part of patient care.” A Person-Centered Visitor Policy After receiving her letter, Renown leadership called Mrs. David Randolph to thank her, offer his sympathies and ask if Renown could help in any way. Darlene asked if he might reconsider allowing families to visit hospital patients during treatment for COVID-19. As the COVID-19 situation has evolved, the policy has as well. Renown hospitals and medical practices now encourage limited visitors for all patients, including those diagnosed with COVID-19. Renown also has extra safety measures to protect the health of patients, visitors and healthcare employees. Darlene is very pleased that her letter inspired this shift in visitor policies for patients with COVID-19. She says, “I have always tried to think of ways I could help other families. Especially those senior couples where one has been hospitalized and the other is home. My wish is to help others.” Renown Health Visitor Policy Renown Health patients may identify two healthy adult “patient supporters” to accompany them on their hospital stay. For more details, visit our Patient Supporter Guidelines page.
Read More About Reno Widow Inspires New Visitor Policy for Renown
-
Pharmacists Answer Questions about the COVID-19 Vaccines
Vaccines that provide protection against the COVID-19 virus are bringing us closer to the end of this deadly pandemic. Two different COVID-19 vaccines are currently available in the U.S. today: one from Pfizer and the other from Moderna. Kate Ward, PharmD, BCPS, Director of Clinical Pharmacy at Renown Health and Adam Porath, PharmD, Vice President of Pharmacy at Renown, share what you need to know about these vaccines. When two COVID-19 vaccines were approved by the U.S. Food & Drug Administration (FDA) in December 2020, it was cause for celebration. Why? Because according to the CDC, the vaccines are 94 percent or more effective in providing protection against the COVID-19 virus! Many people are seeking information about the new Moderna and Pfizer vaccines. Below, our pharmacy leaders provide answers to some commonly asked questions. How do the COVID-19 Vaccines Work? The Pfizer and Moderna vaccines are both mRNA vaccines that help your immune system develop antibodies against the COVID-19 virus. The vaccines use messenger RNA, or mRNA, to show our bodies’ protein-making cells how to make the spike proteins of the COVID-19 virus. Our immune system reacts to these spike proteins by creating antibodies that can recognize and destroy them. So when a person is exposed to the virus in the future, they will be less likely to get sick. What are the Differences between the Pfizer and Moderna Vaccines? The Pfizer and Moderna COVID-19 vaccines are very similar, with just a few small differences worth noting. The main difference between the two vaccines is when you should receive your follow-up dose. Patients who receive a first dose of Pfizer should receive their second dose about three weeks later. Those who receive a first dose of Moderna should receive their follow-up vaccination roughly four weeks after their first dose. People 18 years and older can receive the Moderna vaccine while people 16 years and older can receive the Pfizer vaccine. Dosage for the Moderna vaccine is 0.5 ml (100 mcg). Dosage for the Pfizer vaccine is 0.3 ml (30 mcg).
Read More About Pharmacists Answer Questions about the COVID-19 Vaccines
-
COVID-19 Vaccine Expert Advice
With front-line workers receiving the first COVID-19 vaccinations, many of us are feeling hope, but also worry. As a result, we are joining with the Ad Council, the COVID Collaborative, HHS, CDC and NIAID (along with top health and medical organizations) to address your vaccine concerns and questions. Will the vaccine be available to everyone in Nevada? The Nevada Department of Health and Human Services (DHHS) is collaborating with health systems about the use of initially available, limited supplies of COVID-19 vaccines. They will provide guidance on the prioritization order of who will receive the vaccine. This will be based on available quantities, high-risk locations of work and certain other risk factors, and recommendations and guidance for public health agencies. The CDC has provided guidance to initially focus on the following groups: Healthcare personnel likely to be exposed to or treat people with COVID-19, nursing home residents and others in institutional settings; People at risk for severe illness from COVID-19 due to underlying medical conditions; People 65 years of age and older; Other essential workers. I worry the vaccine has been rushed The U.S. national vaccine safety system ensures that all vaccines are as safe as possible, and because vaccines are given to millions of healthy people to prevent serious diseases, they’re held to very high safety standards. COVID-19 vaccines are undergoing a rigorous development process that includes vaccinating tens of thousands of people who participate in a study to generate the needed clinical data. These clinical trials generate scientific data for the FDA to determine the safety and efficacy of each vaccine. It’s worth noting that the clinical studies to establish the safety and efficacy of the Covid-19 vaccines were as big and thorough as recent studies for other licensed vaccines (for example, the shingles vaccine). I'm concerned about the vaccine's side effects The most common side effects are very similar to those seen with most vaccines, such as: sore arms, fevers, and tiredness within 72 hours after the vaccine. These side effects usually mean that the vaccine is generating an immune response, indicating it is working. Short-term side effects observed in the leading COVID-19 vaccine trials include: Injection site pain and redness Fatigue Muscle aches and pains Joint pain Headache I’m afraid I’ll get COVID-19 from the vaccine None of the authorized and recommended COVID-19 vaccines, or COVID-19 vaccines currently in development in the United States, contain the live virus that causes COVID-19. This means that a COVID-19 vaccine cannot make you sick with COVID-19. Can children receive the COVID-19 vaccine? Not at the moment. In early clinical trials for various COVID-19 vaccines, only non-pregnant adults at least 18 years of age participated. However, clinical trials continue to expand those recruited to participate. The groups recommended to receive the vaccines could change in the future. As of now, it is recommended that children do not receive the vaccine. More information will be available from the vaccine manufacturers. I do not believe vaccines are effective Both this disease and the vaccine are new. We don’t know how long protection lasts for those who get infected or those who are vaccinated. What we do know is that COVID-19 has caused very serious illness and death for a lot of people. If you get COVID-19, you also risk giving it to loved ones who may get very sick. Getting a COVID-19 vaccine is a safer choice. The FDA is responsible for making sure that, just like any other medications, any FDA-authorized or approved COVID-19 vaccines are safe and they work. The EUA (Emergency Use Authorization) will not be provided if the FDA feels that the vaccine is unsafe. I can't get vaccines to due to a medical condition Adults of any age with certain underlying medical conditions are at increased risk for severe illness from the virus that causes COVID-19. mRNA COVID-19 vaccines may be administered to people with underlying medical conditions provided they have not had a severe allergic reaction to any of the ingredients in the vaccine. The following information aims to help people in the groups listed below make an informed decision about receiving the mRNA COVID-19 vaccine. It is extremely important to speak with your doctor regarding your specific medical condition, and always follow their strict advice regarding the COVID-19 vaccine, or any other vaccines. Sources: Renown COVID-19 Ad Council COVID Collaborative U.S. Department of Health & Human Services Centers for Disease Control and Prevention National Institute of Allergy and Infectious Disease
-
Monkeypox: A Renown Expert Weighs In
Renown Health is closely following the national outbreak of the monkeypox virus and urging healthcare providers to be alert for patients with illnesses associated with a rash. In working with the Washoe County Health District (WCHD), Renown is closely monitoring the spread of monkeypox in the community and looking to prevent and reduce the spread of monkeypox. To help to ease worries, we consulted with Paul De Leon, Infection Preventionist at Renown Health. What Exactly is Monkeypox? Monkeypox is a rare viral illness caused by the monkeypox virus — the same family of viruses that causes Smallpox. Although symptoms are similar to Smallpox, monkeypox symptoms are milder and rarely fatal. However, it's important to mention that this virus can be more severe for these susceptible groups: Immunocompromised Pregnant women A fetus or newborn baby Women who are breastfeeding Young children Those with severe skin diseases such as eczema How is Monkeypox Transmitted? The monkeypox virus is not easily transmitted but occurs through sustained person to person close contact with an infected individual. Monkeypox can also be transmitted through direct contact with infectious rash, scabs, or body fluids. Monkeypox can also be spread through prolonged intimate physical contact, such as kissing, cuddling or sex. Lastly, monkeypox can be spread through contaminated linens or bedding. Transmission through respiratory secretions is uncommon but has been reported after prolonged face-to-face contact with symptomatic individuals. In addition, pregnant women can spread the virus to their fetuses through the placenta. Monkeypox Testing If you think you have monkeypox, contact your primary care physician or other medical providers to obtain testing. Notify the provider ahead of time before entering the physical office. Signs & Symptoms This current outbreak of West African monkeypox does not have the typical presentation of classic monkeypox. Symptoms usually appear one to three weeks after infection and include: Pimple-like rash or blisters on the face, inside the mouth, and on other areas of the body, like the hands, feet, chest, genitals, or anus. The rash will go through serval stages, including scabs, before healing and may be painful or itchy. Other symptoms of monkeypox can include: Fever Headache Muscle aches and backache Swollen lymph nodes Chills Exhaustion Respiratory symptoms such as sore throat, nasal congestion, or cough Symptoms of monkeypox may occur before or after a rash with some individuals only report experience a rash. Individuals with monkeypox are infectious once symptoms begin and remain infectious until lesions form scabs, scabs fall off, and a fresh layer of skin forms. The illness typically lasts 2-4 weeks.
-
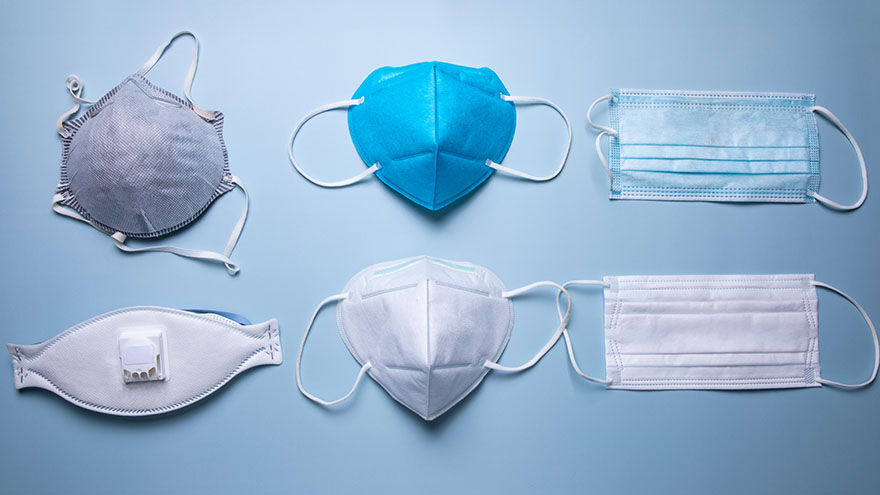
Avoid Counterfeits and Find the Right Protective Mask with This Helpful Guide
To better protect our patients, visitors and employees, cloth masks are no longer allowed at Renown facilities. Surgical masks, KN95 and N95 masks are allowed at Renown facilities. Appropriate face masks will be provided for visitors and patients who need one. With recent surges in the infectious COVID-19 omicron variant, many have sought to upgrade their face masks. But, let’s face it, shopping for face masks with adequate protection can be a challenge, especially considering the countless variations and the rise of counterfeit masks. Follow our straightforward guide below which includes some common red flags to help you discern between a high-quality face mask that provides proper protection and those that may be counterfeit. Types of Masks Qualities of a Real N95 Respirator Mask According to the National Institute for Occupational Safety and Health (NIOSH), N95 approved masks form a tight seal around your face and include a disposable respirator that removes particles including bacteria, viruses and dust as you breathe. N95 masks that are NIOSH approved undergo strict quality assurance and performance requirements to ensure mask respirators filter out up to 95% of hazardous particles. As a rule of thumb, N95 masks will not have ear loops, commonly used in cloth or surgical face masks. N95 respirators will contain two elastic bands or head straps that fasten behind the head, one securing the crown of the head and the other resting at the base of the neck, providing a snug fit and seal. Some other common signs that an N95 might be counterfeit include lack of all proper labeling, misspellings of NIOSH, decorative fabric and claiming to be approved for children. Currently, masks in adult sizes are the only masks to undergo NIOSH’s quality assurance and testing process. Respirators approved by NIOSH will include a testing certification (TC) approval number and will contain specific labeling on the facepiece of your mask. Find a full list of Center for Disease Control (CDC) and NIOSH requirements here. Identifying Real KN95 Respirator Masks Often preferred due to comfortability, the KN95 respirators were initially designed to meet Chinese standards for medical masks. Firstly, if a KN95 mask claims to be approved by the CDC, it is counterfeit as the CDC and NIOSH do not support any respiratory protective devices according to international standards. However, when KN95 masks are fitted and worn appropriately they do provide more protection than disposable masks. Legitimate KN95 masks will display a manufacturer number, GB2626-2019, ensuring accordance with current Chinese respirator standards for all masks made after July 1, 2021. Unlike N95 masks, it is important to note that KN95 masks are available in children's sizes. You might run into KN95 masks claiming to be “FDA (Food and Drug Administration) approved” or “FDA-registered,” but be aware that this does not mean much and is a misleading statement. What this indicates is that a mask maker has submitted paperwork to the FDA, but the product has not been thoroughly tested for proper filtration and protection. Surgical Masks Medical procedure masks often referred to as surgical or disposable masks vary in their protection according to a variety of factors including fit and filtration. The CDC defines medical procedure masks as “variably shaped, including flat pleated, cone-shaped, or duck-bill. Medical procedure masks are loose and are not expected to provide a reliable level of protection against airborne or aerosolized particles as N95 respirators regulated by the National Institute of for Occupational Safety and Health.” However, these types of masks provide more protection than cloth masks and are certainly better than wearing no mask at all. Often popular due to their level of comfort and cost-effectiveness, surgical masks can be knotted in the ear loop areas to provide a tighter seal and can be layered for additional filtration. Depending upon your budget and level of comfortability and protection, one variation of mask may suit you over another. Please remember to do your part in limiting the spread of COVID-19 and wear a surgical mask, KN95 or N95 mask when visiting Renown facilities.
Read More About Avoid Counterfeits and Find the Right Protective Mask with This Helpful Guide